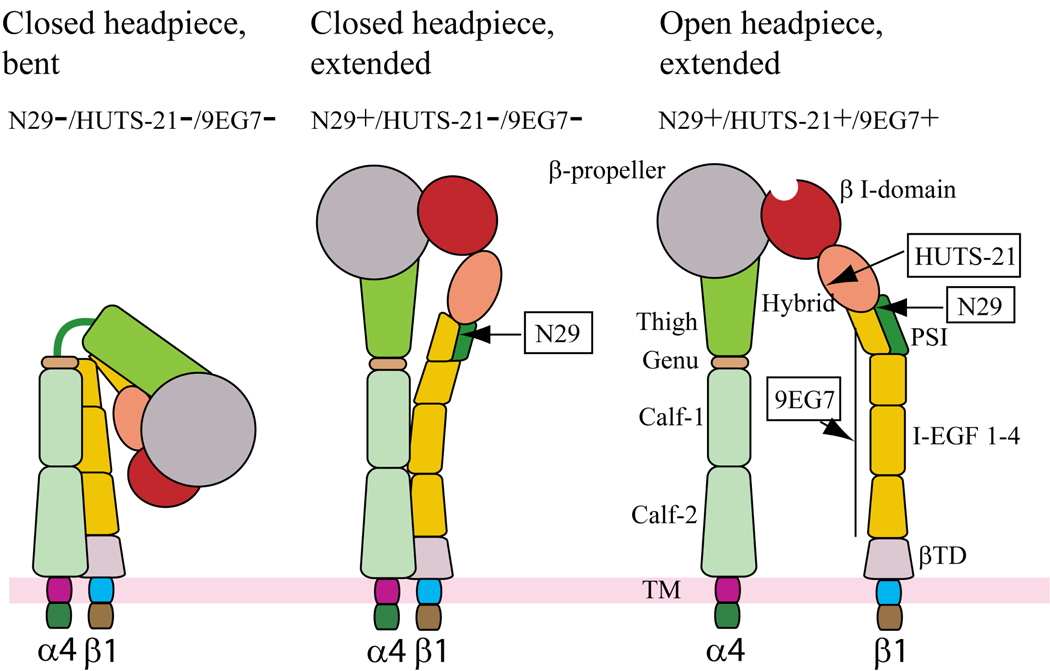
Figure 3. Antibody probes of α4β1 conformation and affinity.
Schematic of three putative conformations of α4β1 that might be expressed by eosinophils as modeled on the crystal structures of αVβ3 and αIIbβ3 and adapted from Luo BH et al. [164]. The bent, closed form of α4β1 is presumably not recognized by any of the activation-sensitive antibodies (left). Extension of α4β1 reveals the epitope in the β1 PSI domain recognized by N29 - this conformation is presumed to most closely depict the conformation of α4β1 of blood or airway eosinophils (middle). Further activation results in swing-out of the hybrid domain and exposure of the epitope recognized by HUTS-21, along with separation of the integrin legs and exposure of the epitope recognized by 9EG7 (right). The latter form of α4β1 is presumably present on Jurkat, EoL-3, or Mn2+- or PMA-activated AML14.3D10 eosinophilic cells [30].