Abstract
Background and Objectives
Obesity is a chronic disease that requires good eating habits and an active life style. Obesity may start in childhood and continue until adulthood. Severely obese children have complications such as diabetes, hypercholesterolemia, hypertension and atherosclerosis. The goal of this study was to determine the effects of exercise programs on anthropometric, metabolic, and cardiovascular parameters in obese children.
Subjects and Methods
Fifty four obese children were included. Anthropometric data such as blood pressures, body mass index (BMI) and obesity index (OI) were measured. Blood glucose, total cholesterol, triglycerides, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), high sensitive-CRP (hs-CRP), brachial-ankle pulse wave velocity (BaPWV) and ankle brachial index (ABI) were measured. Physical fitness measurements were done. Obese children were divided into three groups: an aerobic exercise group (n=16), a combined exercise group (n=20), and a control group (n=18). Obese children exercised in each program for 10 weeks while those in the control group maintained their former lifestyle. After 10 weeks, anthropometric data and cardiovascular parameters were compared with the data obtained before the exercise program.
Results
LDL-C, waist circumference, and systolic blood pressure decreased significantly in the aerobic exercise group compared to the control group (p<0.05). Waist circumference and systolic blood pressure decreased significantly in the combined exercise group compared to controls (p<0.05). Physical fitness level increased significantly after the exercise programs (p<0.05 vs. control). PWV did not show a significant change after exercise.
Conclusion
A short-term exercise program can play an important role in decreasing BMI, blood pressure, waist circumference, LDL-C and in improving physical fitness. Future investigations are now necessary to clarify the effectiveness of exercise on various parameters.
Keywords: Obesity, Exercise, Cardiovascular disease
Introduction
As the degree of obesity increases, the prevalence of metabolic syndrome increases. Metabolic syndrome often begins in childhood. Moreover, research using noninvasive measures of peripheral vascular morphology and function shows a relationship between subclinical atherosclerosis and cardiometabolic risk factors in childhood.1-3) Abdominal obesity and insulin resistance are directly related both clinically and epidemiologically to the development of metabolic syndrome and to an increase in cardiovascular risk.
The combination of dietary and exercise interventions appears to provide the most beneficial effects. Behavioral modification in overweight children reduces body weight, improves body composition, and positively modifies many of the components of the metabolic syndrome.4) Similar beneficial effects have been observed for endothelial dysfunction, with the greatest advancements occurring when combined dietary and exercise interventions are used in overweight children.5)
An early intervention aimed at managing obesity could reduce the risk of developing metabolic syndrome. It is conceivable that even in the absence of weight loss, overweight and obese children may improve their cardiovascular risk profile by lifestyle changes and therapies targeted toward each component of the syndrome.6)
Exercise training can improve insulin sensitivity and endothelial vascular function as well as glycemic control and blood pressure.7) However, there have been few studies of changes of cardiovascular parameters and physical strength after exercise programs in obese children.
The purposes of this study were to compare anthropometric and cardiovascular parameters before and after an exercise program and to evaluate physical fitness level after the exercise program.
Subjects and Methods
Subjects
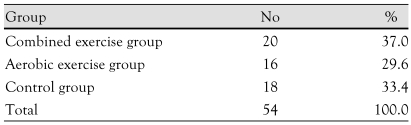
Fifty four obese children (45 males, 9 females) aged 12 to 14 participated in this study (Table 1). The body mass index (BMI) for each individual was over the 95th percentile for age and gender. They were divided into three groups consisting of an aerobic exercise group (n=16), a combined exercise group (n=20), and a control group (n=18) (Table 1). All participants gave written informed consent. This study was approved by the Institutional Review Board of Ewha Womans University Hospital. All subjects were evaluated before and 10 weeks after the exercise program.
Table 1.
The number of subjects in each exercise group
Anthropometric measurement
Blood pressure data were recorded by averaging the two blood pressure measurements made after 5 minutes of rest with an oscillometric monitor. If the blood pressure was high, the measurement was done again using a mercury manometer. Careful attention to cuff size was made to avoid overdiagnosis of high blood pressure. The cuff completely encircled the upper part of the arm to ensure uniform compression and the inflatable bladder covered at least 2/3 of the upper arm length and 80-100% of its circumference. We included only patients who had never been diagnosed or treated for hypertension before. Height, waist circumference and body weight were measured and BMI and obesity index (OI) were calculated. BMI was defined as weight (kg) divided by height squared (m2). The OI was calculated by the equation below using the standard weight as the value corresponding to the 50th percentile of the weight data chart for Korean children.
OI (%)=(weight measured-standard weight)/standard weight×100
Obesity was defined as an OI above 120%. Fat mass and fat distribution were measured by bioelectrical impedance analysis (Inbody 3.0, Biospace, Seoul, Korea).
Brachial-ankle pulse wave velocity and ankle brachial index
Brachial-ankle pulse wave velocity (BaPWV) and ankle brachial index (ABI) were measured by a VP-1000 instrument (Colin Co., Komaki, Japan). Using a volume plethysmographic technique, PWV, ABI (the ratio of systolic blood pressure in the ankle to that in the brachial artery), blood pressure of both extremities, electrocardiography and heart sounds were obtained simultaneously. Cuffs were wrapped on both arms and ankles, and electrocardiogram electrodes were placed on the left sternal border. As the pulse wave contours of the four extremities were recorded, the cuffs inflated and deflated automatically. Cuffs were attached to the plethysmographic sensor which determined the volume pulse form. Blood pressure was measured by the oscillometric pressure sensor. BaPWV was determined by the pulse transit time and the distance between these two segments. The distance between segments was calculated automatically, based on the height of the subjects. All measurements were made during regular sinus rhythm.
Metabolic parameters in the blood
Venous blood was drawn from all children after overnight fasting. Samples were kept at -70℃ for subsequent assay. Serum concentrations of glucose, total cholesterol, triglycerides, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), aspartate aminotransferase, alanine aminotransferase and high sensitive-CRP (hs-CRP) were evaluated.
Physical fitness assessment
We assessed the physical fitness level of subjects in five different ways. Time to run one mile was measured before and after the exercise program. Trunk flexion strength was determined as follows. The subjects took off their shoes and sat on the trunk flexion measurement instruments with two feet touching the perpendicular surface of the instruments. Directed by instructors, the participants flexed the trunk with their hands pushing the scale, and the instructor read the scale at the maximum stretch position. Curl-up was performed for 1 minute and long jump was also performed. Grip strength was measured. The participants performed the grip test for both hands twice alternatively for a total of 4 times and the maximum grip strength was recorded.
Exercise program
The combined exercise program consisted of warm-up exercise, main exercise and cool-down exercise. During the warm-up phase, the children were directed to stretch and jog for 5 minutes. Body weight, circuit weight training, and aerobic exercise were included in the main exercise program. Circuit weight training consisted of 8-10 kinds of aerobic and resistance training exercise. The subjects were directed to do each exercise for 30 seconds with 10 seconds rest intervals between each. Exercise intensity was adjusted to be moderate (70-80% of maximum strength). Children were directed to exercise three times a week, each time including two circuit weight training routines and one aerobic exercise routine. The duration for each exercise session was 60 minutes. During the cool-down phase, children stretched for 5 minutes.
The aerobic exercise program consisted of warm-up exercise, main exercise, and cool-down exercise. The warm-up phase and the cool-down phase were the same as for the combined exercise program. The main exercise phase consisted of various items that can trigger the interest of children, such as soccer, basketball, football, baseball, hockey, badminton, healthrobics, rope skipping, and mountain climbing. Exercise intensity was adjusted to a VO2max of 60-80%, a HRmax of 70-90%, and 300-400 kcal for one exercise session. Children were directed to exercise three times a week, twice with an instructor and once by themselves. The duration for each exercise session was 60 minutes. In the cool-down phase children did stretching for 5 minutes.
Statistical analysis
We performed all statistical analyses using an Statistical Package for the Social Sciences (SPSS)/PC software package (SPSS version 11.0) program. Descriptive statistics are presented as means and standard deviations. The comparison of continuous variables was done using the Student t-test or one-way analysis of variance. A p less than 0.05 was considered as statistically significant.
Results
Anthropometric data
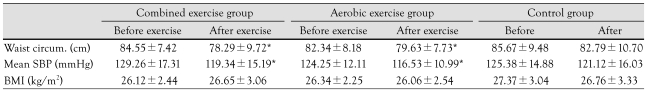
The baseline anthropometric data in all three groups before the exercise were similar. Waist circumference (84.55±7.42 cm vs. 78.29±9.72 cm in combined exercise group, 82.34±8.18 cm vs. 79.63±7.73 cm in aerobic exercise group) and systolic blood pressure (129.26±17.31 mmHg vs. 119.34±15.19 mmHg in combined exercise group, 124.25±12.11 mmHg vs. 116.53±10.99 mmHg in aerobic exercise group) decreased significantly after the exercise program (Table 2). Height increased in both the combined and aerobic exercise groups but there was no significant difference between these two groups. BMI (Table 2), OI, fat mass and fat distribution did not change significantly after exercise.
Table 2.
Anthropometric data before and after exercise in obese and control groups
*p<0.05 before exercise vs. after exercise. SBP: systolic blood pressure, BMI: body mass index
Brachial-ankle pulse wave velocity and ankle brachial index
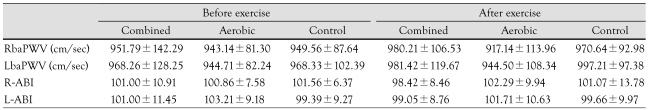
PWV and ABI were not significantly different before and after exercise, or between groups (Table 3).
Table 3.
Comparison of PWV and ABI among groups before and after exercise program
All parameters were not significantly changed after exercise program. RbaPWV: right brachial-ankle pulse wave velocity, LbaPWV: left brachial-ankle pulse wave velocity, R-ABI: right ankle-brachial index, L-ABI: left ankle-brachial index
Blood test
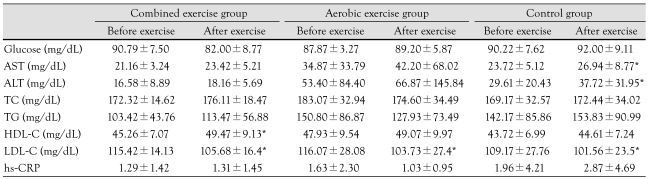
HDL-C levels increased significantly (45.26±7.07 mg/dL vs. 49.47±9.13 mg/dL) only in the combined exercise group. LDL-C decreased (115.42±14.13 mg/dL vs. 105.68±16.43 mg/dL) after exercise program in the combined exercise group. In the aerobic exercise group, LDL-C decreased (116.07±28.08 mg/dL vs. 103.73±27.48 mg/dL) after the exercise program. There was no statistically significant difference between groups for LDL-C (Table 4).
Table 4.
Comparison of blood test parameters among groups before and after an exercise program
*p<0.05 before exercise vs. after exercise. AST: aspartate aminotransferase, ALT: alanine aminotransferase, TC: total cholesterol, TG: triglyceride, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, hs-CRP: high sensitive-C reactive protein
Physical fitness measurements
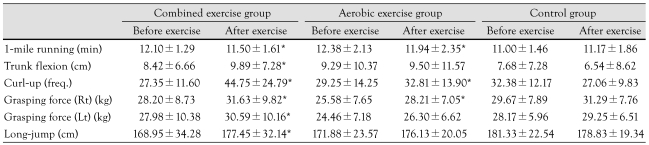
One mile running, curl-up, grip strength, and long jump increased significantly after either the combined or the aerobic exercise program (Table 5). Trunk flexion increased significantly after the combined exercise program (Table 5). No significant changes in physical fitness measurements were noted in the control group. VO2max increased in all three groups (Table 6).
Table 5.
Change in physical strength after an exercise program
*p<0.05 before exercise vs. after exercise. min: minute, freq.: frequency, Rt: right, Lt: left
Table 6.
Comparison of VO2 max among groups
No: number
Discussion
In this study, we demonstrated that an exercise program is effective in treating children's obesity. Regular exercise, which means combined and aerobic exercise, decreases BMI, blood pressure, waist circumference and LDL-C, which are the causes of metabolic syndrome. Exercise also increases physical fitness level, which directly reflects health status.3)
Childhood obesity has been associated with elevated blood pressure, elevated triglycerides,9) low HDL-C,9) abnormal glucose metabolism,10) insulin resistance,11) inflammation,12-14) and compromised vascular function.13) Obesity progresses from childhood to adulthood, and childhood adiposity is a strong predictor of obesity, insulin resistance,14) and abnormal lipids in adulthood.15) Moreover, the rate of increase in adiposity during childhood is significantly associated with the development of cardiovascular risk in young adults. Metabolic syndrome with obesity occurs in 38.7% of moderately obese (BMI 33.4 kg/m2 and 49.7% of severely obese (BMI 40.6 kg/m2 children and adolescents.3)
In order to prevent this progression from childhood obesity to adult obesity and metabolic syndrome, we need to instruct children to exercise regularly. Regular exercise has a positive influence in prevention and treatment of obesity such as decreasing body weight and insulin resistance, which are the causes of metabolic syndrome.16) Aerobic exercise has been reported to be associated with lower risk of cardiovascular mortality.8) Recently the need for estimates of overweight and obesity in children to assess preventive measures, monitor secular trends, and identify high risk groups has been emphasized.3),8)
Physical activity is also associated with lower levels of inflammatory cytokines and markers of oxidative stress.17) High participation in physical activity is positively correlated with insulin sensitivity in adolescents18) and with improved endothelial function and HDL-C, even in the absence of weight loss.19) Many of the controlled intervention studies addressing this issue have shown that exercise improves adipokine and oxidative stress levels; however, most of these trials have reported concomitant improvements in body weight or composition that occurred during the exercise training period. Changes in body weight composition confound the data with regard to the direct effects of exercise on these variables because adipocytes are the main mediators of the hormones. Exercise improves adipokines and inflammatory markers in adults and children20) independent of weight loss.
CRP has gained a great deal of attention from the medical community because its concentration is a significant predictor of the incidence of cardiovascular disease and because it has prognostic value among adults with existing cardiovascular disease.21) Because cardiovascular disease often has its origins in childhood and because several risk factors for cardiovascular disease progress from childhood to adulthood, understanding the distribution and implications of risk factors such as hs-CRP among children is of considerable interest. According to the National Health and Nutrition Examination Survey of 1999-2000, BMI is the most consistent and strongest predictor of hs-CRP concentration.22) In our study, we investigated hs-CRP, but there was no significant change after either exercise program. This may be because the duration of the exercise program was too short to bring about a change.
Both endothelial function and arterial stiffness are improved after exercise training in adult studies.23) However PWV did not show a significant change in our study. Different effects of exercise training program can be seen depending on the duration of the exercise program. Longer-term exercise programs, ones lasting 12 weeks, demonstrated improvements in endothelial function in adult individuals with diabetes and impaired glucose tolerance.24) Short-term exercise programs have been shown to improve glucose tolerance without weight loss25) and 4-week-programs improve arterial function in individuals with pre- or mild hypertension.26) Such short-term programs are useful to investigate the effects of exercise without the confounder of weight loss.
In our study, a short-term exercise program for 10 weeks played an important role in decreasing BMI, blood pressure, waist circumference, LDL-C and in improving physical strength. However, parameters such as PWV and hs-CRP did not change. This may be due to the short study duration, which was only 10 weeks. Another limitation of our study is the small number (n=54) of subjects. A larger sample size would increase statistical power and a longer longitudinal study would reduce the problem of false negative conclusions.
In summary, we found significant differences among children in waist circumference, blood pressure, LDL-C and physical strength after an exercise program. Further investigations are necessary to clarify the effectiveness of exercise in children on various parameters.
Acknowledgments
This work was supported by Korean Heart Foundation.
References
- 1.Bouchard L, Drapeau V, Provencher V, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. 2004;80:1478–1486. doi: 10.1093/ajcn/80.6.1478. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Eckel RH. Obesity and cardiovascular disease. Curr Atheroscler Rep. 2002;4:448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 3.Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 4.Lissin LW, Gauri AJ, Froelicher VF, Ghayoumi A, Myers J, Giacommini J. The prognostic value of body mass index and standard exercise testing in male veterans with congestive heart failure. J Card Fail. 2002;8:206–215. doi: 10.1054/jcaf.2002.126812. [DOI] [PubMed] [Google Scholar]
- 5.Woo KS, Chook P, Yu CW, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 6.Cha BS, Kim HJ. Metabolic syndrome and cardiovascular disease. Korean Circ J. 2003;33:645–652. [Google Scholar]
- 7.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- 8.Evenson KR, Stevens J, Thomas R, Cai J. Effect of cardiorespiratory fitness on mortality among hypertensive and normotensive women and men. Epidemiology. 2004;15:565–572. doi: 10.1097/01.ede.0000129527.53181.c8. [DOI] [PubMed] [Google Scholar]
- 9.Backman L, Freyschuss U, Hallberg D, Melcher A. Reversibility of cardiovascular changes in extreme obesity: effects of weight reduction through jejunoileostomy. Acta Med Scand. 1979;205:367–373. doi: 10.1111/j.0954-6820.1979.tb06066.x. [DOI] [PubMed] [Google Scholar]
- 10.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 11.Alpert MA, Lambert CR, Panayiotou H, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76:1194–1197. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 12.Karason K, Lindroos AK, Stenlof K, Sjostrom L. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000;160:1797–1802. doi: 10.1001/archinte.160.12.1797. [DOI] [PubMed] [Google Scholar]
- 13.Drenick EJ, Fisler JS. Sudden cardiac arrest in morbidly obese surgical patients unexplained after autopsy. Am J Surg. 1988;155:720–726. doi: 10.1016/s0002-9610(88)80029-1. [DOI] [PubMed] [Google Scholar]
- 14.Hong YM, Koo HS, Lee JY, Jung JW, Kim NS, Noh CI. Serum cytokine levels in hypertensive children: tumor necrosis factor-alpha, interleukin-6. Korean Circ J. 2007;37:312–317. [Google Scholar]
- 15.Iacobellis G, Ribaudo MC, Leto G, et al. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res. 2002;10:767–773. doi: 10.1038/oby.2002.104. [DOI] [PubMed] [Google Scholar]
- 16.Barbeau GR, Arsenault F, Dugas L, Simard S, Lariviere MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J. 2004;147:489–493. doi: 10.1016/j.ahj.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Eisenstein EL, Shaw LK, Nelson CL, Anstrom KJ, Hakim Z, Mark DB. Obesity and long-term clinical and economic outcomes in coronary artery disease patients. Obes Res. 2002;10:83–91. doi: 10.1038/oby.2002.14. [DOI] [PubMed] [Google Scholar]
- 18.Lakka TA, Lakka HM, Salonen R, Kaplan GA, Salonen JT. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–504. doi: 10.1016/s0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 19.Voors AW, Webber LS, Frerichs RR, Berenson GS. Body height and body mass as determinants of basal blood pressure in children: the Bogalusa Heart Study. Am J Epidemiol. 1977;106:101–108. doi: 10.1093/oxfordjournals.aje.a112439. [DOI] [PubMed] [Google Scholar]
- 20.Otsuka Y, Miyazaki S, Okumura H, et al. Abnormal glucose tolerance, not small vessel diameter, is a determinant of long-term prognosis in patients treated with balloon coronary angioplasty. Eur Heart J. 2000;21:1790–1796. doi: 10.1053/euhj.2000.2181. [DOI] [PubMed] [Google Scholar]
- 21.de Maat MP, Kluft C. Determinants of C-reactive protein concentration in blood. Ital Heart J. 2001;2:189–195. [PubMed] [Google Scholar]
- 22.Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2003;108:1053–1058. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- 23.Baynard T, Carhart RL, Jr, Ploutz-Snyder LL, Weinstock RS, Kanaley JA. Short-term training effects on diastolic function in obese persons with the metabolic syndrome. Obesity. 2008;16:1277–1283. doi: 10.1038/oby.2008.212. [DOI] [PubMed] [Google Scholar]
- 24.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cononie CC, Goldberg AP, Rogus E, Hagberg JM. Seven consecutive days of exercise lowers plasma insulin responses to an oral glucose challenge in sedentary elderly. J Am Geriatr Soc. 1994;42:394–398. doi: 10.1111/j.1532-5415.1994.tb07487.x. [DOI] [PubMed] [Google Scholar]
- 26.Collier SR, Kanaley JA, Carhart R, Jr, et al. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–686. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]