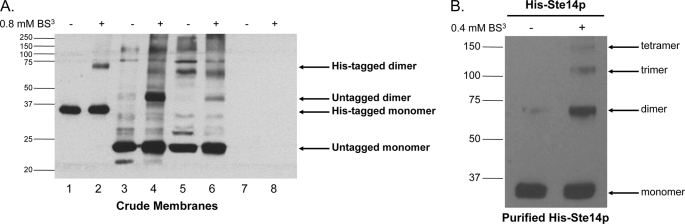
FIGURE 1.
Immunoblot of His-Ste14p cross-linked chemically with BS3. A, 80 μg of crude membrane protein were incubated with 0.8 mm BS3 for 20 min at room temperature. The reaction was terminated by the addition of SDS-PAGE sample buffer without reducing agents and then heated for 20 min at 65 °C. Various amounts of crude membrane proteins were separated by 10% SDS-PAGE, and His-Ste14p was detected using a Ste14p polyclonal antibody (1:1,000) and a HRP-conjugated secondary goat anti-rabbit antibody (1:10,000) as follows: lane 1, 1 μg of His-Ste14p (CH2704); lane 2, 1 μg of His-Ste14p (CH2704) + BS3; lane 3, 10 μg of untagged Ste14p (CH2866; 2μ PGK promoter); lane 4, 10 μg of untagged Ste14p (CH2866; 2μ PGK promoter) + BS3; lane 5, 50 μg of SM3495 (untagged Ste14p; 2μ STE14 promoter); lane 6, 50 μg of SM3495 (untagged Ste14p; 2μ STE14 promoter) + BS3; lane 7, 50 μg of CH2714 (negative control); lane 8, 50 μg of CH2714 (negative control) + BS3. Protein bands were visualized using enhanced chemiluminescence (ECL). B, purified His-Ste14p (2.5 μg) was incubated with 0.4 mm BS3 for 20 min, and the reaction was terminated by the addition of SDS-PAGE sample buffer without reducing agents. The reactions were heated for 30 min at 65 °C, 0.1 μg of purified protein was separated by 7.5% SDS-PAGE, and the protein bands were visualized by immunoblot analysis as described above.