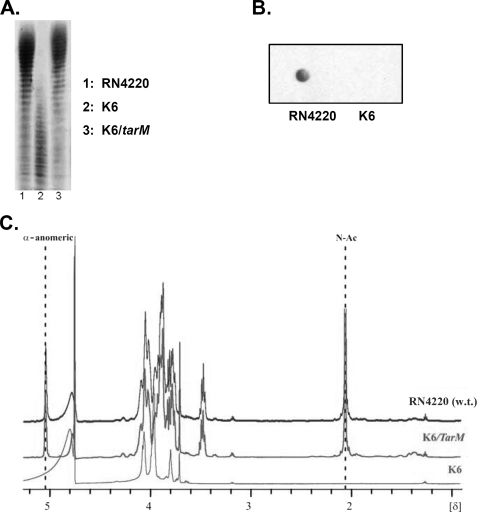
FIGURE 3.
tarM disruption leads to altered WTA that lacks the α-GlcNAc residues. A, PAGE analysis of WTA is shown. The indicated WTA samples (100 nmol of phosphate/lane) were resolved in polyacrylamide gels and visualized by Alcian blue silver staining. The PAGE migration behaviors of WTA from wild type (lane 1) and tarM-complemented mutant strain (lane 3) were almost the same; the tarM mutant WTA migrated much faster (lane 2) and exhibited weaker staining with Alcian blue-silver solution. B, purified WTA spotted on a nitrocellulose membrane was incubated with GlcNAc binding biotinylated WGA and visualized with streptavidin-HRP. In contrast to wild-type WTA, mutant WTA could not be stained with WGA, indicating the loss of GlcNAc from the K6 WTA backbone. C, 1H NMR spectrum of WTA from RN4220 derivatives is shown. The two characteristic signals for GlcNAc at δ 5.07 and 2.08 (anomeric proton of α-GlcN and methyl group of NAc, respectively) were found and are indicated in the spectra of wild type and the tarM complement (K6/tarM) WTA but were missing in the spectrum of mutant K6 WTA, indicating that disruption of tarM in the mutant K6 leads to the loss of the α-GlcNAc from WTA and that α-GlcNAc modification could be restored by complementation with a plasmid encoding tarM.