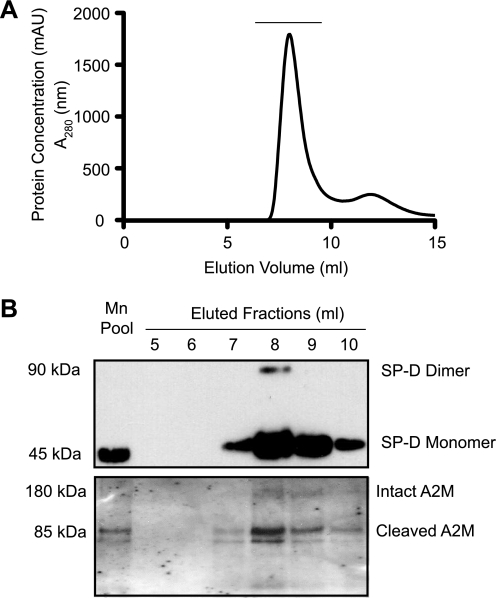
FIGURE 3.
SP-D and A2M co-purify from BALF. A, SP-D present in the BALF was captured by maltose-agarose affinity chromatography in the presence of calcium ions, competitively eluted with manganese ion, and the proteins present in the manganese elution pool (Mn Pool) were further separated by a Superose 6 gel exclusion column. B, Western blot showing that SP-D and A2M co-purify from BALF. Fractions underlined in A (from elution at 5–10 ml, 1-ml fractions) were subjected to Western blots for SP-D and A2M. The major protein present under this peak was SP-D. Intact and cleaved A2Ms were also detected in these fractions. Some surfactant protein A (SP-A) and gp-340 were also seen in the same fractions (data not shown).