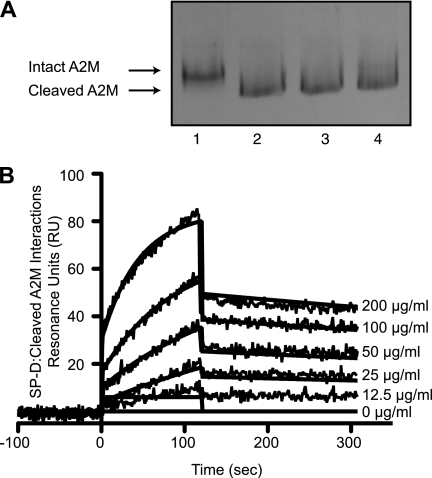
FIGURE 7.
Cleaved A2M binds well to SP-D. A, methylamine causes thiol ester cleavage-induced conformational changes in A2M. Incubation with methylamine converted A2M (2 μg) from its slow form (lane 1) to its fast form (lanes 2–4) as can be seen via Coomassie Blue-stained native polyacrylamide gel. Lane 1, A2M; lane 2, A2M + 100 mm methylamine; lane 3, A2M + 40 mm methylamine; lane 4, A2M + 10 mm methylamine. B, surface plasmon resonance sensorgram shows immobilized SP-D (20 μg/ml) on a GLC chip binding to methylamine-cleaved A2M (200 μg/ml in a 2-fold serial dilution) in the presence of 5 mm CaCl2. A2M was washed out at 120 s. The KD for this interaction is 8.54 × 10−9 indicating interactions will occur in the nanomolar range. The KD for intact A2M was 7.33 × 10−9 m (Fig. 2B). Curves were modeled using the Langmuir binding model. These results confirm that SP-D would bind cleaved A2M similar to that of intact A2M.