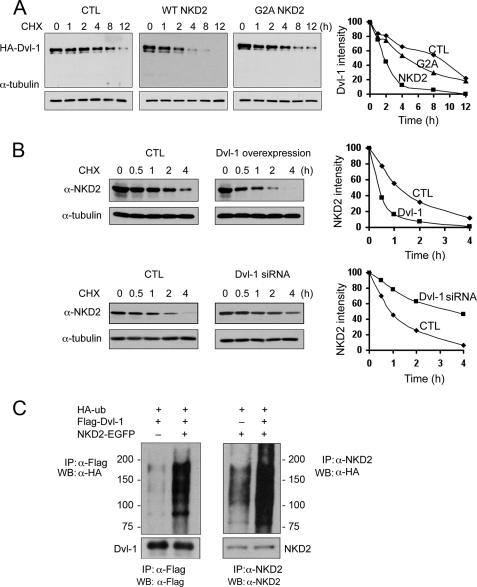
FIGURE 4.
NKD2 and Dvl-1 accelerate each other's degradation via polyubiquitylation. A, MDCK cells were transfected transiently with HA-Dvl-1 and wild-type NKD2-EGFP or G2A NKD2-EGFP. Cycloheximide (CHX, 10 μg/ml) was added for the times indicated to block protein synthesis. Dvl-1 stability was monitored by Western blotting. α-Tubulin served as a loading control. Densitometry was performed, and the intensity of Dvl-1 band was determined after normalization to α-tubulin. B, MDCK cells stably expressing wild-type NKD2-EGFP were transfected transiently with HA-Dvl-1 and control vector (upper panel) or Dvl-1 siRNA and control vector (lower panel). The stability of NKD2 was determined over time following the administration of CHX (10 μg/ml). Levels of NKD2 and α-tubulin were monitored by Western blot analysis. Densitometry was performed, and the intensity of the NKD2 band was determined after normalization to α-tubulin. In A and B, protein levels were normalized to α-tubulin and graphed with time = 0 arbitrarily set to 100%. C, increased ubiquitylation of Dvl-1 (left) or NKD2 (right) in HEK293 cells transiently expressing HA-Ub, FLAG-Dvl-1, or wild-type NKD2. HEK293 cells were treated with MG132 (50 μm) for 4 h before the ubiquitylation assay.