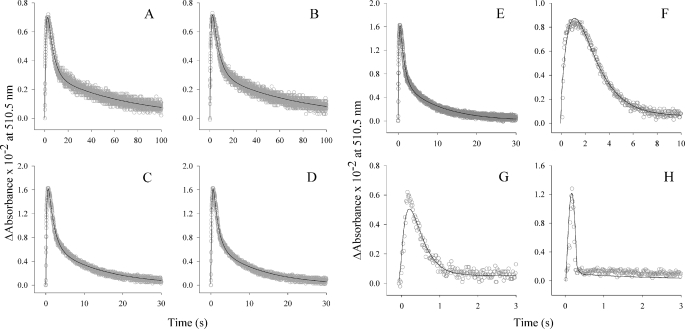
FIGURE 3.
The single turnover reactions of isolated hyperactive ALAS variants. The presteady state kinetic parameters were determined under single turnover conditions (60 μm enzyme-glycine complex and 10 μm succinyl-CoA) at 20 °C by monitoring absorbance changes at 510 nm. The variant-catalyzed reactions A4, D8, G7, A8, F3, and F10 (A–F, respectively) resemble that of the wild-type enzyme in that quinonoid lifetime is observed as a single kinetic step assigned to quinonoid intermediate formation and a two-step kinetic process assigned to its decay (9). The reaction kinetics of the F1 and H1 variants (G and H, respectively) are markedly altered in that the second of the two kinetic steps assigned to quinonoid intermediate decay is not observed.