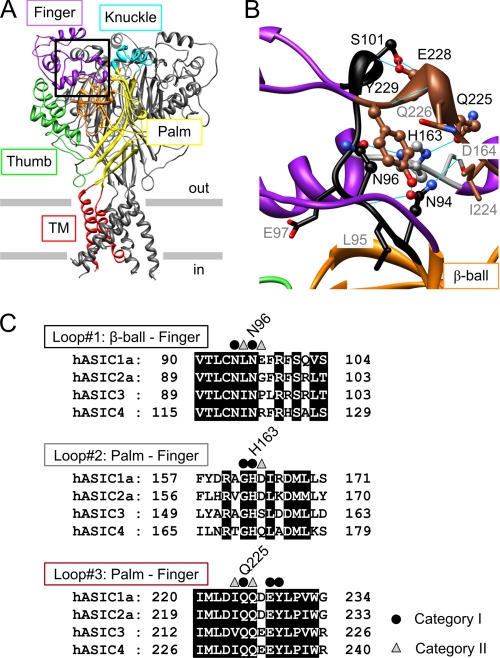
FIGURE 1.
Structure and conservation of the finger-ball interaction zone in ASIC1a. A, structure of hASIC1a established by homology modeling based on chicken ASIC1 (Protein Data Bank code 2QTS). One of the three ASIC subunits is shown in color, identifying different parts of ASIC subunits (red, transmembrane segments; yellow, palm; orange, β-ball; green, thumb; purple, finger; turquoise, knuckle). B, view of the finger-ball interaction zone with loop 1 in black, loop 2 in gray, and loop 3 in brown. The side chains of the residues mutated in this study are represented as “ball-and-stick” (category I residues, see text) and as “stick” (category II residues). Hydrogen bonds predicted in Chimera (33) are indicated as blue solid lines. A and B are presented in stereo view in supplemental Fig. S1. C, sequence alignment of the three loops forming the finger-ball interaction zone, highlighting the amino acids substituted in this study. The amino acid sequences of the extracellular regions of human ASIC1a, -2a, -3, and -4 are aligned (GenBankTM accession numbers are as follows: hASIC1a, 21536349; hASIC2a, 1280439; hASIC3, 3747101; and hASIC4, 8346834). Category I residues are marked by ● and category II residues by ▵.