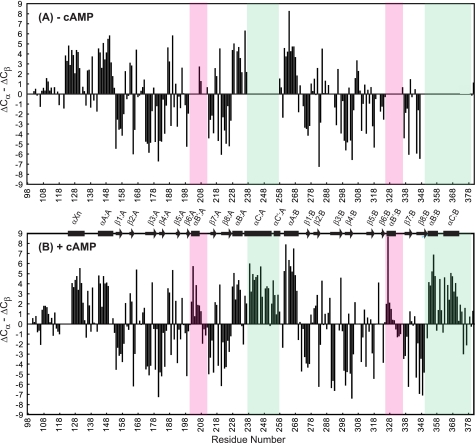
FIGURE 3.
Cα and Cβ secondary chemical shift data for RIα-(98–381). Secondary chemical shifts of the apoprotein (A) and cAMP-bound protein (B) are plotted versus residue number. The PBC regions are boxed in pink, and helices αC:A, αC′:A, αB:B, and αC:B are marked by green boxes. ΔCα and ΔCβ are calculated by subtracting the Cα and Cβ shifts from random coil values (46) for the residues whose chemical shifts were assigned unambiguously. α-Helices and β-strands present in the x-ray crystal structure of cAMP-bound bovine RIα-(92–379) (10) are depicted by rectangles and arrows, respectively.