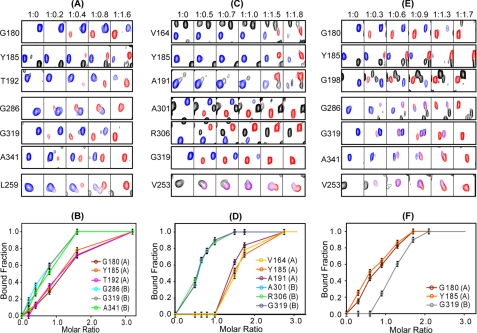
FIGURE 5.
Binding of cAMP and cyclic nucleotide analogs to RIα-(98–381). Representative resonances from residues residing in the A and B domains as well as the linker helices (αC:A and αC′:A) were monitored throughout the titration with cAMP (A and B), 2-Cl-8-AH-cAMP (C and D), and N6-MB-cAMP (E and F). A, C, and E, ligand-free RIα-(98–381) resonances are colored in blue, and ligand-bound resonances are shown in red. Resonances arising from intermediate bound conformations are shown in purple (see details under “Results”). The molar ratios for RIα-(98–381) and ligand are listed at the top. Titration curves for cAMP (B), 2-Cl-8-AH-cAMP (D), and N6-MB-cAMP (F) are also shown where the bound fraction was obtained using the spectra in A, C, and E, respectively, by dividing the intensities of the bound resonances (red) by the sum of the intensities of the bound (red) and free (blue) resonances. The CNB domains A or B, to which the residues belong are indicated in parenthesis next to the residue identification in B, D, and F.