Abstract
In the domestic fowl and other oviparous vertebrates, estrogens induce hepatic synthesis of yolk proteins. The oviduct also increases in size and produces ovalbumin when estrogens are administered. DNA-RNA hybridization assays indicate that within 105 minutes following treatment with estrone the liver of immature pullets contains most, if not all, of the liver RNA species that are present in the livers of the laying hen. Because of limitations of the nucleic acid hybridization technique, which remain to be clearly defined, it is not known whether the hepatic RNA populations in estrone-treated pullets and laying hens are completely homologous or differ in some important way. The results indicate that the genomic response in the avian liver to exogenous estrone is „normal” (relative to the laying hen) and further suggest that the hepatic response to estrogen is primarily pertinent to vitellinogenesis.
DNA-RNA hybridization assays on total RNA also indicate that estrogen-induced RNA species in the liver are not homologous to estrogen-evoked RNA species of the oviduct. Therefore, in these two target organs estrogen-evoked RNA synthesis appears to be in part organ-specific. These data indicate that the specificity of hormone action can be explained in part on the basis of induced synthesis of specific RNA molecules. Whether these alterations in transcription are due to indirect hormonal action or are the result of a primary action of estrogens or estrogen-binding site complexes on the genome and/or its repressors remains a basic question.
Full text
PDF

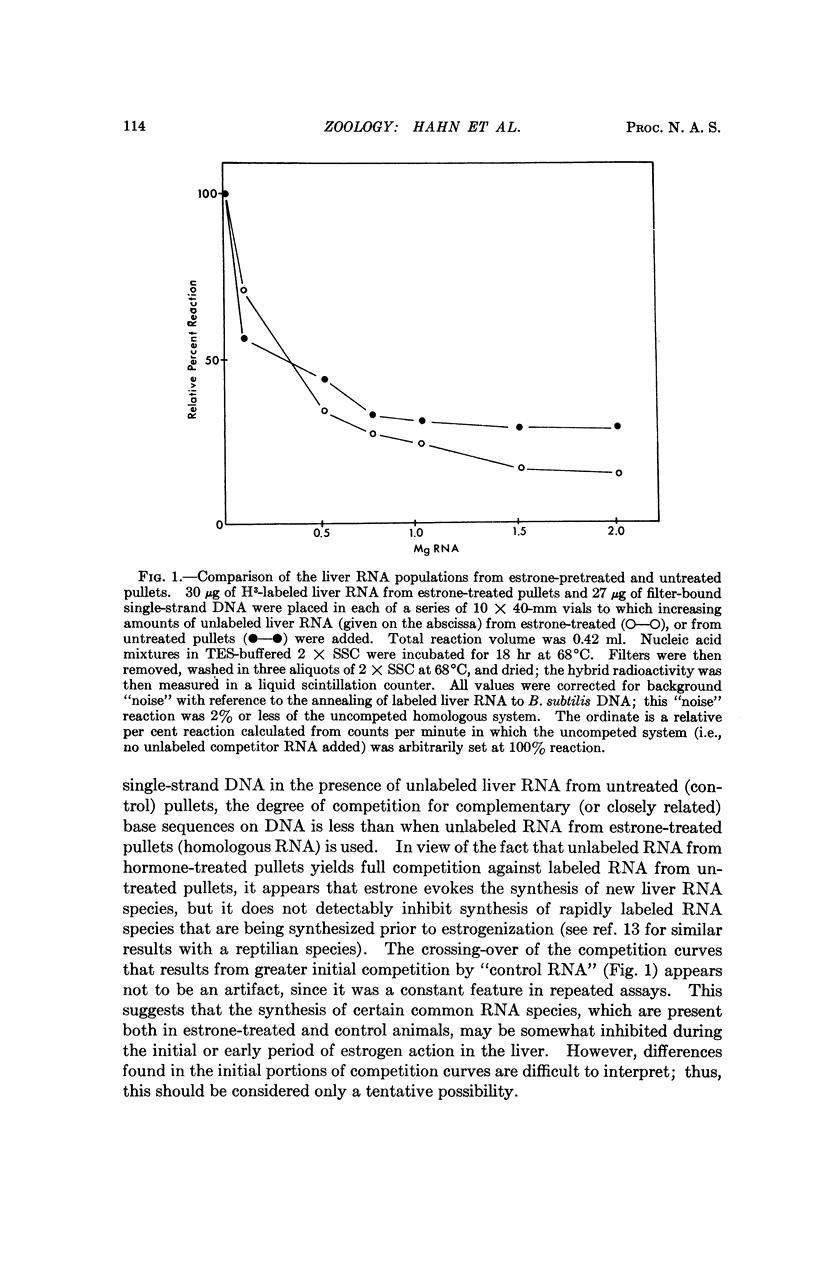
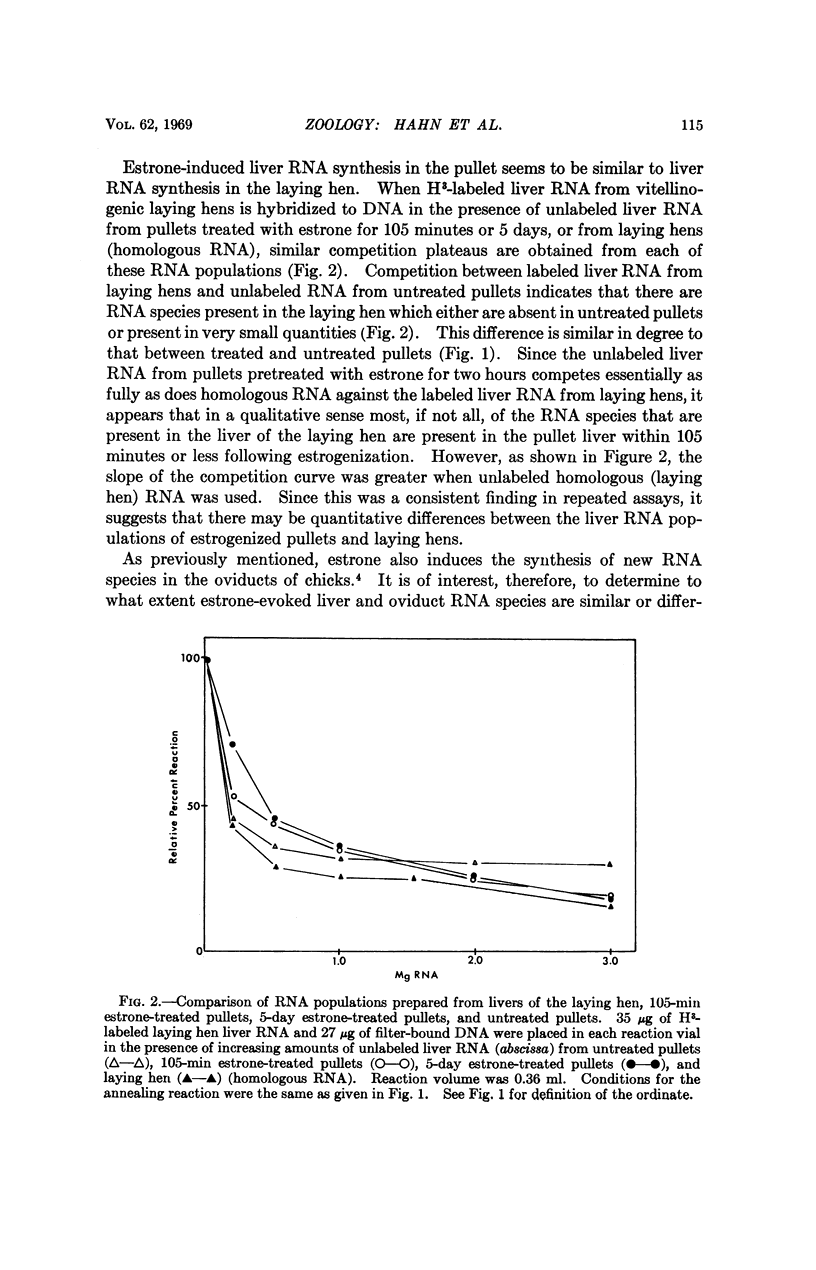
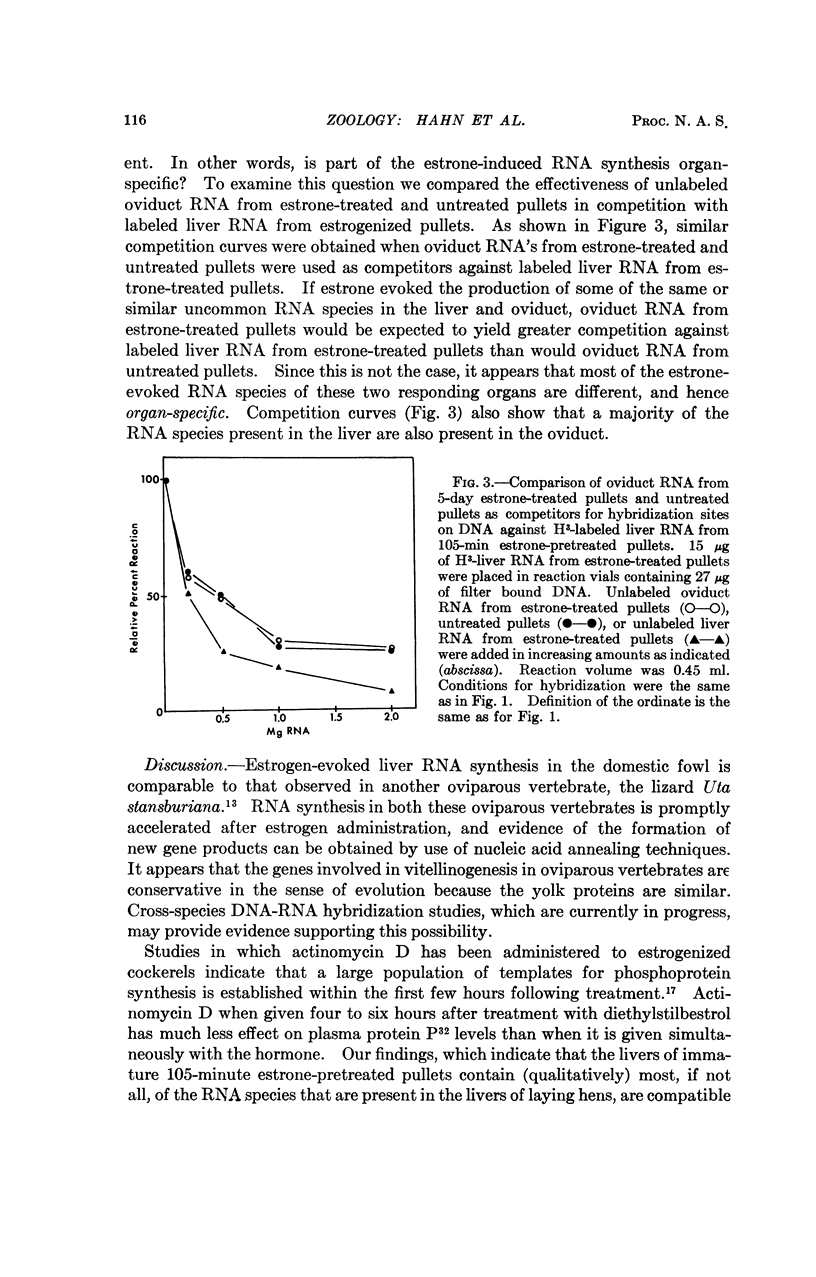



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Church R., McCarthy B. J. Changes in nuclear and cytoplasmic RNA in regenerating mouse liver. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1548–1555. doi: 10.1073/pnas.58.4.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- FLICKINGER R. A., ROUNDS D. E. The maternal synthesis of egg yolk proteins as demonstrated by isotopic and serological means. Biochim Biophys Acta. 1956 Oct;22(1):38–42. doi: 10.1016/0006-3002(56)90220-7. [DOI] [PubMed] [Google Scholar]
- GREENGARD O., GORDON M., SMITH M. A., ACS G. STUDIES ON THE MECHANISM OF DIETHYLSTILBESTROL-INDUCED FORMATION OF PHOSPHOPROTEIN IN MALE CHICKENS. J Biol Chem. 1964 Jun;239:2079–2082. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hahn W. E., Church R. B., Gorbman A., Wilmot L. Estrone- and progesterone-induced synthesis of new RNA species in the chick oviduct. Gen Comp Endocrinol. 1968 Jun;1(3):438–442. doi: 10.1016/0016-6480(68)90055-5. [DOI] [PubMed] [Google Scholar]
- Hahn W. E. Estradiol-induced vitellinogenesis and concomitant fat mobilization in the lizard Uta stansburiana. Comp Biochem Physiol. 1967 Oct;23(1):83–93. doi: 10.1016/0010-406x(67)90475-6. [DOI] [PubMed] [Google Scholar]
- Houssais J. F., Attardi G. High molecular weight nonribosomal-type nuclear RNA and cytoplasmic messenger RNA in HeLa cells. Proc Natl Acad Sci U S A. 1966 Aug;56(2):616–623. doi: 10.1073/pnas.56.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. O., Grimley P. M., O'Malley B. W. Protein synthesis: differential stimulation of cell-specific proteins in epithelial cells of chick oviduct. Science. 1968 Apr 5;160(3823):86–87. doi: 10.1126/science.160.3823.86. [DOI] [PubMed] [Google Scholar]
- Korner A. Ribonucleic acid and hormonal control of protein synthesis. Prog Biophys Mol Biol. 1967;17:61–98. doi: 10.1016/0079-6107(67)90004-1. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Chalkley G. R. Some properties of a nuclear binding site of estradiol. J Mol Biol. 1967 Aug 14;27(3):431–441. doi: 10.1016/0022-2836(67)90049-6. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J. Arrangement of base sequences in deoxyribonucleic Acid. Bacteriol Rev. 1967 Dec;31(4):215–229. doi: 10.1128/br.31.4.215-229.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady J. E. The determination of oestradiol and oestrone in the plasma of the domestic fowl by method involving the use of labelled derivatives. Biochem J. 1968 Jan;106(1):77–86. doi: 10.1042/bj1060077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Korenman S. G. Estrogen stimulation of synthesis of specific proteins and RNA polymerase activity in the immature chick oviduct. Biochim Biophys Acta. 1967 Aug 22;145(1):204–207. doi: 10.1016/0005-2787(67)90679-x. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Middleton P. A. Altered gene expression during differentiation: population changes in hybridizable RNA after stimulation of the chick oviduct with oestrogen. Nature. 1968 Jun 29;218(5148):1249–1251. doi: 10.1038/2181249a0. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Brandt J., Miller I. Hydrocortisone-induced inhibition of protein synthesis and uridine incorporation in isolated bone cells in vitro. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1599–1606. doi: 10.1073/pnas.57.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. T. Molecular hybridization used to characterize the RNA synthesized by isolated bovine thymus nuclei. Proc Natl Acad Sci U S A. 1968 Mar;59(3):846–853. doi: 10.1073/pnas.59.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Estrogen induces lipophosphoprotein in serum of male Xenopus laevis. Science. 1968 Apr 5;160(3823):91–92. doi: 10.1126/science.160.3823.91. [DOI] [PubMed] [Google Scholar]


