Abstract
Neuropathic pain remains a serious medical problem because of patient morbidity and the absence of effective therapeutic interventions. Recent evidence suggests that this type of pain may be particularly difficult to manage because underlying mechanisms are influenced by a variety of factors, including type of injury, site of injury, and time after injury. This situation is exacerbated by the fact that different mechanisms may contribute to unique aspects of neuropathic pain, including ongoing pain as well as mechanical and thermal hypersensitivity. The different ion channels present in primary afferent neurons implicated in each of these aspects of neuropathic pain are reviewed.
Introduction
Neuropathic pain negatively impacts the lives of millions, and treatment or prevention represents a major, worldwide, unmet medical need. Neuropathic pain also remains one of the most challenging problems faced by clinicians who can offer their patients few, if any, consistently effective means of relief devoid of deleterious side effects. Neuropathic pain most commonly arises from injury or disease of the peripheral nervous system. Clinical data on the therapeutic efficacy of local and regional peripheral nerve blocks and surgical interventions, in combination with results from microneurographic analysis of neuropathic pain patients, suggest that neuropathic pain reflects the emergence of aberrant activity in peripheral nerves [1]. Biochemical/molecular biological analysis of peripheral nerve removed from neuropathic pain patients and a wealth of data from preclinical neuropathic pain models suggest that aberrant afferent activity reflects changes in the expression, biophysical properties, and/or distribution of ion channels [1]. Specific patterns of ion channel changes in specific subpopulations of afferents are thought to account for most, if not all, of the so-called positive signs of peripheral neuropathy, the most troubling of which include ongoing pain, dynamic mechanical allodynia, cold allodynia, and heat hyperalgesia [2]. However, because a discussion of all the ion channel changes that have been described in primary afferent neurons in response to nerve injury or disease is beyond the scope of this review, we describe patterns of ion channel changes that are thought to account for the various positive signs of neuropathic pain within the context of factors, including type of injury, site of injury, and time after injury, that influence patterns of ion channel changes.
Although this review focuses on the primary afferent, it is important to emphasize that following peripheral nerve injury, there are changes in the central nervous system (CNS) that contribute to the manifestations of neuropathic pain. These changes have both a quantitative and qualitative impact, influencing the intensity and area of perceptual changes [3], as well as the emergence of novel sensory phenomena such as dynamic mechanical allodynia [4]. It also is clear that recapitulating several of the changes that have been described within the CNS, such as activation of microglia, can induce peripheral nerve injury–like changes in behavior [5]. What remains a topic of active debate, however, is the extent to which changes within the CNS alone are sufficient for the manifestation of neuropathic pain. We suggest that the weight of evidence from both clinical and preclinical studies indicates that aberrant afferent activity is necessary for the initiation and maintenance of all aspects of neuropathic pain associated with injury to the peripheral nervous system.
Factors Influencing Underlying Mechanisms
Type and site of injury
Neuropathic pain has many diverse etiologies (Table 1). Consistent with the suggestion that the type of injury influences underlying mechanisms, patients may describe very different positive signs depending on the cause of nerve damage. For example, most diabetic polyneuropathy patients describe a burning pain in the lower extremities (typically the feet) [6••]. However, fewer patients suffering from trigeminal neuralgia or postamputation pain report the presence of burning pain [6••]. Following amputation, there is a higher prevalence of pain paroxysms (electric shock–like or stabbing pain) compared with diabetic polyneuropathy, postherpetic neuralgia, or radiculopathy [6••]. Furthermore, although there are trigger points associated with trigeminal neuralgia, none are associated with the metabolic neuropathic pain disorders, and whereas some neuropathic pain syndromes (beriberi, diabetic neuropathy) are associated with a “stocking-glove” progression of pain suggestive of a dying back of peripheral nerve fibers, others are not associated with this phenomenon [6••]. Importantly, although traumatic injury to a trigeminal nerve, traumatic injury to a somatic nerve, and diabetic neuropathy are all associated with changes in voltage-gated Na+ channels (VGSCs), the pattern of changes differs in each case.
Table 1.
Types of neuropathic pain
| Metabolic | Beriberi, diabetic polyneuropathy |
| Traumatic | Nerve crush, transection |
| Infectious | Postherpetic neuralgia, HIV neuropathy |
| Toxic | Paclitaxel-mediated polyneuropathy |
| Hereditary | Familial amyloid neuropathy |
| Immune-mediated | Multiple sclerosis, vasculitic neuropathy |
Time after injury
Following injury to a peripheral nerve, there are also time-dependent changes in symptomatology and underlying mechanisms. For example, diabetic neuropathy has a typical pattern, progressing centrally from the distal extremities where it is first manifest in a stocking-glove pattern in association with damage to nerves with the longest axons [6••]. Following complete transection of the sciatic nerve, spontaneous activity is first detected in Aβ-fibers, presumably those previously innervating low-threshold receptors; it is only after a considerable delay that spontaneous activity is detected in C fibers [1]. At a mechanistic level, there appear to be time-dependent changes in the contribution of the VGSC, Nav1.8, to various aspects of neuropathic pain, where it plays a critical role in one population of afferents at early time points and a different population of afferents at later time points after nerve injury [7]. Highlighting the complexity of the problem and the fact that changes in a single class of ion channels is insufficient to account for all aspects of neuropathic pain, there is evidence that nerve injury–induced changes in Na+ currents do not always correlate with changes in excitability [1].
Mechanisms underlying several nerve injury–induced time-dependent changes in ion channels and consequently afferent excitability have been described. This issue has been most extensively studied in preclinical models of partial nerve injury in which two distinct processes impact distinct populations of afferents. By definition, a partial nerve injury will result in 1) injured afferents with axons disconnected from their original target of innervation at the point of injury, and 2) the “uninjured” neighbors of the injured afferents. Axons distal to the site of injury are broken down and reabsorbed by immune cells via a process referred to as Wallerian degeneration. This process can take up to a week to complete and is associated with the release of inflammatory mediators, many of which act on afferents to modulate ion channel properties and afferent excitability [8]. Innervation density is maintained, at least in part, by competition for a limited supply of neurotrophic factors such as nerve growth factor (NGF) and artemin that are released from peripheral tissue. The partial denervation of peripheral tissue results in an increase in neurotrophic factors available to remaining or uninjured fibers [1]. Changes in access to neurotrophic factors have both short- and long-term consequences, in which an increase in neurotrophic factor initiates second messenger pathways resulting in the modulation of ion channel properties on a time scale of seconds to minutes, as well as changes in the pattern of ion channel expression that are manifest on a time scale of many hours to days [1]. Because the increase in inflammatory mediators and neurotrophic factors surrounding the uninjured afferents soon after nerve injury is similar to that observed in the presence of inflammation, many of the changes observed in uninjured afferents following a partial nerve injury are similar to those observed in the presence of inflammation where the specific pattern of changes is determined, at least in part, by the peripheral tissue.
In contrast to the inflammation-like changes that develop in uninjured afferents following a partial nerve injury, the injured afferents undergo a different pattern of changes driven, at least in part, by the loss of access to neurotrophic factors normally supplied by the tissue previously innervated. For example, administration of NGF to the distal end of a cut nerve reverses both the increase in Nav1.3 and decrease in Nav1.8 expression observed in the absence of NGF [9]. Glial-derived neurotrophic factor (GDNF) has been shown to prevent the nerve injury–induced decrease in Nav1.8 and Nav1.9 [10]. If these trophic factor–dependent changes in sodium channel expression are mediated by the high affinity receptors for NGF and GDNF—tyrosine receptor kinase type A (TrkA) and GDNF family receptor α1 (GFRα1), respectively—they should be restricted to a subpopulation of putative nociceptive afferents based on the expression pattern of the receptors. GDNF, in particular, has been shown to attenuate both nociceptive behavior [11] and spontaneous activity in injured afferents [1], despite evidence that spontaneous activity develops in low-threshold, presumably TrkA and GFRα1 negative afferents [12]. These observations suggest that either the antinociceptive actions of GDNF are mediated through another presumably low affinity receptor, or the effect is indirect, implying a link between the changes in nociceptive afferents and the emergence of spontaneous activity in non-nociceptive afferents. Importantly, time-dependent changes in trophic factors and inflammatory mediators, for example, and the resultant change in the biophysical properties, expression, and/or distribution of ion channels may underlie time-dependent changes in the efficacy of certain drug therapies.
Genetic background
A firm link between genes and the likelihood of developing neuropathic pain has yet to be established in humans, with large-scale, genomewide association studies only now underway. Nevertheless, a compelling body of evidence from preclinical models suggests that genes influence the magnitude of pain behavior observed in the presence of neuropathy. For example, there are large mouse strain differences in the magnitude of mechanical hypersensitivity that develop following lumbar nerve root injury [13]. Similar results have been reported in rats in several models of traumatic nerve injury [14]. The genetic mechanisms that predispose to neuropathic pain warrant further investigation as they may provide novel targets for drug development and increase our understanding of the underlying pathophysiology.
Transduction to Transmitter Release
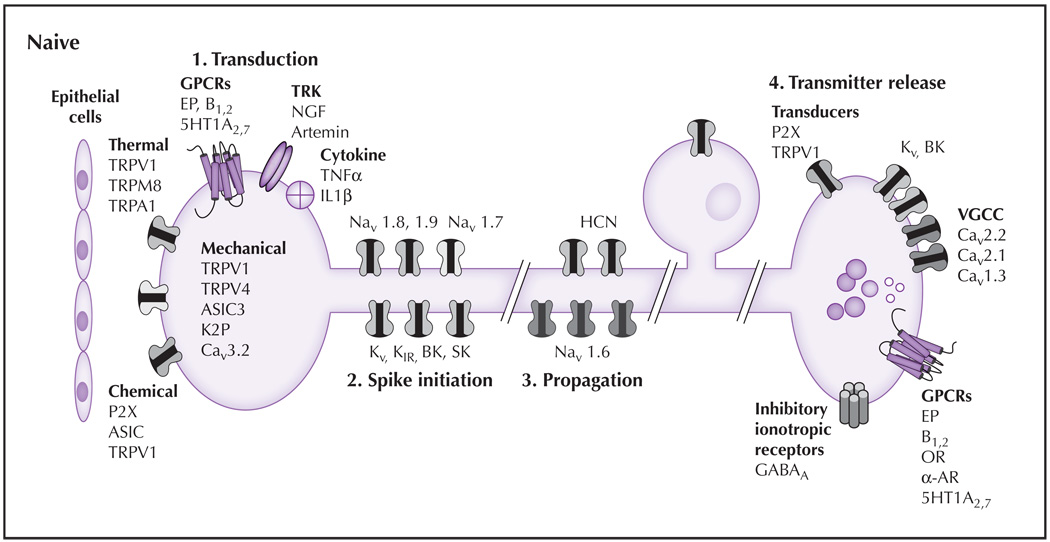
To evaluate the relative impact of nerve injury–induced changes in ion channels on the expression of neuropathic pain, it is useful to first consider mechanisms of nociceptive signaling in the absence of tissue injury. The ability to respond to noxious stimuli is a vital adaptive mechanism that alerts the body to danger and prevents the potential for further tissue damage. Alterations in these normal processes are the basis for aberrant activity and neuropathic pain. Afferent signaling depends on four general processes: 1) signal transduction (conversion of thermal, mechanical, and chemical stimuli into a membrane depolarization referred to as a generator potential); 2) action potential initiation; 3) action potential propagation; and 4) neurotransmitter release (Fig. 1 and Fig. 2).
Figure 1.
The principal sensory functions of nociceptive afferents consist of transduction, spike initiation, propagation, and transmitter release. (1) Transduction: In naive tissue, proteins thought to play a role in mechanotransduction include TRPV4, ASIC-3, and Cav3.2. Several different classes of TRP channels are involved in transduction of temperature from noxious cold (TRPA1), cool (TRPM8), warm (TRPV4), and hot (TRPV1). Ionotropic chemoreceptors present in nociceptive afferents include TRPV1, ASIC3, and P2X3. A wide variety of metabotropic receptors also are present on the terminals of nociceptive afferents including GPCRs, which are responsive to prostaglandins (EP, bradykinin, 5HT), TRK, and receptors for cytokines. (2) Spike initiation: Action potential threshold is regulated by several K+ channels (eg, Kv, KIR, K2P, BK, SK). HCN channels also contribute to action potential threshold. Nav1.9 also may contribute to establishing action potential threshold. The channels responsible for the upstroke of the action potential include Nav1.7 and Nav1.8. (3) Spike propagation: The ion channels underlying action potential propagation are distinct from those underlying spike initiation and include Nav1.6. HCN channels also contribute to spike propagation. (4) Transmitter release: The release of transmitter at the central terminals of nociceptive afferents is dependent on VGCCs, which are modulated following activation of inhibitory GPCRs and serve as the primary mechanism for the therapeutic efficacy of OR and α-AR agonists. Transmitters present in nociceptive afferents are generally packaged in small clear vesicles (which generally contain the excitatory amino acid glutamate) and large dense core vesicles (which contain, among other things, neuropeptides [eg, substance P, calcitonin generelated peptide]). A number of excitatory transducers (eg, P2X3 and TRPV1) appear to facilitate central transmitter release. Inhibition of the central terminal may involve activation of Kv, BK channels, and presynaptic GABAA receptors. Excitatory GPCRs (eg, EP, B1,2 receptors) also are present. 5HT—serotonin; AR—adrenergic receptor; ASIC—acid-sensing ion channel; B—bradykinin; BK—large conductance Ca2+ dependent channel; GABA—γ-aminobutyric acid; GPCR—G protein–coupled receptor; HCN—hyperpolarization-activated cyclic nucleotide channel; IL—interleukin; NGF—nerve growth factor; OR—opioid receptor; SK—small conductance Ca2+ dependent channel; TNF—tumor necrosis factor; TRK—tyrosine receptor kinases; TRPA—transient receptor potential ankyrin; TRPM—transient receptor potential melastatin; TRPV—transient receptor potential vanilloid; VGCC—voltage-dependent Ca2+ channel.
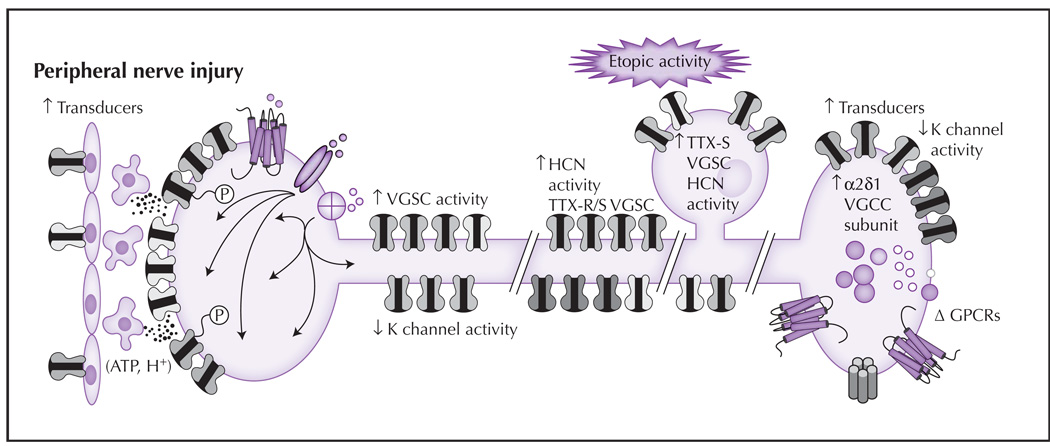
Figure 2.
Following partial nerve injury, changes in nociceptive terminals of the spared or uninjured afferent result in an increase in sensitivity to noxious stimuli and the emergence of membrane depolarization. Similar changes also may occur at sites of damage in the injured afferents, although the mechanisms driving the changes appear to be different. (1) Transduction: There are increases in the density of several transducers and post-translational modifications (depicted as phosphorylation, P) that increase channel activity or sensitivity. These events are facilitated by release of mediators from epithelial cells. (2) Spike initiation: Changes following nerve injury include a decrease in K+ channel density and/or current, and an increase in HCN and Na+ channel density and/or activity. (3) Spike propagation: The pattern of channels influencing propagation can change following injury, including redistribution of Nav1.7 and/or Nav1.8 to the cell membrane. (4) Ectopic activity: In the presence of nerve injury, changes in the soma, sites of injury, and/or other sites along the axon, such as an increase in sodium and HCN channels, may lead to membrane instability (manifest as oscillatory behavior). The aberrant distribution of transducers also has been described. (5) Transmitter release: Changes that occur in the central terminals of nociceptive afferents after nerve injury include an increase in the α2δ1-subunit of VGCCs. This subunit is important for trafficking channels to the membrane. There also is an increase in neuropeptide expression, the emergence of additional excitatory receptors, and a decrease in K+ currents that facilitate nociceptive signaling. ATP—adenosine triphosphate; GPCR—G protein–coupled receptor; HCN—hyperpolarization-activated cyclic nucleotide channel; TTX—tetrodotoxin; VGCC—voltage-dependent Ca2+ channel; VGSC—voltage-gated Na+ channel.
Signal transduction
Nociceptive afferents terminate in free nerve endings, a term used to describe the absence of a specialized cell type at the afferent terminal that is responsible for signal transduction. Recent evidence suggests, in fact, that other cell types may contribute to signal transduction in nociceptive afferents, particularly in the presence of injury [15]. Nevertheless, the presence of free nerve endings suggests that sensory transduction involves processes intrinsic to the afferent terminal. Consistent with this suggestion, there is evidence that nociceptive afferents in isolation are responsive to thermal (both heating and cooling), mechanical, and chemical stimuli [12]. As discussed below, many of the proteins responsible for this process have been identified and are present in afferent terminals.
Members of the transient receptor potential (TRP) family of ion channels provide the molecular basis for thermosensation [16]. These transducer elements are nonselective voltage–dependent cation channels that are activated by changes in temperature. Transient receptor potential vanilloid type 1 (TRPV1) was the first TRP family member identified in this context and appears to play a role in heat sensation, and more specifically, thermal hyperalgesia in the presence of tissue injury [17]. TRPM8 appears to be responsible for the detection of a decrease in temperature (ie, cooling), whereas TRPA1 may contribute to the detection of noxious cold temperatures [16]. Although biophysical data clearly indicate TRPA1 is activated by noxious cold temperatures [18], results from TRPA1 null mutant mice suggest that the channel plays a more significant role in mechanotransduction [19]. Importantly, each of the “thermo-TRP” channels also is activated by both endogenous and exogenous chemical stimuli that may be far more relevant than thermal stimuli as a means of channel activation in the presence of tissue injury [20••].
The molecular mechanisms underlying high-threshold mechanotransduction is less understood, but several different channel types have been implicated, including members of the TRP channel family (TRPV4, TRPA1, TRPC); members of the epithelia Na+ channel (ENaC) superfamily, in particular acid-sensing ion channels (ASICs); members of the two-pore potassium channel (K2P) family, a low-threshold voltage-gated Ca2+ channel; and ionotropic purinergic receptors, in particular P2×3 [20••]. Evidence for the majority of these channels comes from heterologous expression studies in combination with evidence that the channels are present in sensory neurons. Data from null mutant mice are more compelling but suggest that mechanotransduction is likely to involve a complex set of proteins. For example, TRPV4 and TRPA1 knockout mice are less responsive to mechanical stimulation [21]. Furthermore, in visceral afferents, enhancement of TRPV4 signaling increased responses to mechanical stimulation, whereas decreases in TRPV4 had the opposite effect [22]. In ASIC3 knockout mice, the mechanical sensitivity in high-threshold A-fiber nociceptors is decreased, whereas it is increased in low-threshold, rapidly adapting afferents [23]. Finally, P2X receptors appear to contribute to mechanotransduction indirectly: In the bladed, colon, and skin epithelial, cells may transduce mechanical stimuli via yet to be determined mechanisms and then release adenosine triphosphate (ATP), which subsequently activates nearby afferent terminals containing P2X receptors [15].
A wide variety of chemoreceptors are present on sensory neurons, enabling the rapid response to an array of both endogenous and exogenous chemicals. These are generally broken down into ionotropic and metabotropic receptors (several ionotropic receptors described above). The metabotropic receptors include the classical 7-transmembrane G protein–coupled receptors; receptors bearing intrinsic protein tyrosine kinase domains (ie, Trk receptors); receptors that associate with cytosolic tyrosine kinases (ie, nontyrosine kinase receptors such as cytokine receptors and integrins); and protein serine/threonine kinases (ie, transforming growth factor [TGF]-β receptors) [24]. Metabotropic receptors are coupled, directly or indirectly, to a range of ion channels resulting in afferent activation and/or sensitization. These receptors play a critical for the emergence of aberrant activity observed following nerve injury.
Spike initiation and propagation
A variety of voltage-dependent and -independent K+, Na+, Ca2+, and Cl− channels as well as nonselective cation channels appear to influence membrane resistance of the afferent terminal, and therefore the spread of the generator potential and ultimately action potential threshold and afferent excitability. Nevertheless, VGSCs are ultimately responsible for the upstroke of the action potential and are therefore essential for action potential generation and propagation.
VGSCs consist of an α-subunit that contains everything necessary for a functional channel, including voltage sensor, channel pore and inactivation gate, and up to two β-subunits that influence channel gating and trafficking [7]. Nine α-subunits, Nav1.1–Nav1.9, have been cloned and demonstrated to form functional channels, and all but one, Nav1.4, are present in sensory neurons. However, these channels are differentially distributed within a given afferent and between subpopulations of afferents [7]. The tetrodotoxin (TTX)-resistant channels, Nav1.8 and Nav1.9, are preferentially expressed in nociceptive afferents. Nav1.8 is normally targeted to the afferent cell body and terminals [7], whereas Nav1.6 is localized along axons of nociceptive afferents and at nodes of Ranvier in myelinated axons [20••]. Localized at afferent terminals, Nav1.8 is critical for spike initiation in nociceptive afferents. In contrast to all other VGSCs that are inactivated by cooling, Nav1.8 remains functional at noxious cold temperatures, and is, therefore, critical for the detection of cold pain [25]. There also is evidence that Nav1.7 is targeted to afferent terminals and plays an important role in spike initiation as well as establishing the excitability in subpopulations of nociceptive afferents. This has been most clearly illustrated in individuals with point mutations in this channel [26••].
Voltage-gated K+ channels and Ca2+–modulated K+ channels also are critical for spike initiation and propagation, as these channels underlie the repolarizing phase, or downstroke of the action potential and the afterhyperpolarization. These channels also are important in setting firing frequency and properties of the action potential shape (ie, rate of fall, duration, afterhyperpolarization) [20••]. These channels also influence transmitter release secondary to their influence on action potential duration and consequently Ca2+ influx associated with the action potential. The differential distribution of K+ channels within and between afferents contributes to the heterogeneity in firing properties observed.
Neurotransmission
Neurotransmitter release from central terminals is required to convey information from the periphery into the CNS. Neurotransmitter release is dependent on Ca2+ influx mediated by voltage-dependent Ca2+ channels (VGCCs) present in afferent terminals. VGCCs are characterized as being high-voltage activated ([HVA]; N-type: Cav2.2; L-type: Cav1.1, Cav1.2, Cav1.3; R-type: Cav2.3; and P/Q-type: Cav2.1) or low-voltage activated ([LVA]; T-type: Cav3.1, Cav3.2, Cav3.3) [27], but it is the HVA channels (in particular N-type channels) that are responsible for transmitter release from afferent terminals. LVA channels appear to be primarily responsible for influencing the excitability of afferent terminals, with recent evidence suggesting that these channels may be involved in subthreshold oscillations and/or spike initiation in a subpopulation of nociceptive afferents [28].
Ion Channels and the Emergence of Aberrant Activity
Ongoing pain
Ongoing pain is the most common and troubling positive sign associated with neuropathic pain. It is generally assumed that ongoing pain reflects ongoing activity in a subpopulation of nociceptive afferents and/or ongoing activity in a subpopulation of non-nociceptive afferents that as a result of a phenotypic switch (ie, the expression of a nociceptive transmitter) or a change within the CNS, signals pain. A number of mechanisms have been identified that may account for the emergence of ongoing pain. Unfortunately for patients and clinicians alike, all of these mechanisms are likely to contribute to ongoing pain, but the relative contribution of each is likely influenced by the factors discussed above (Fig. 1 and Fig. 2).
Ongoing afferent drive
Many of the mediators released at sites are able to drive action potential generation in nociceptive afferents directly via the activation of both ionotropic and metabotropic receptors. There is evidence of functional receptors, such as TRPV1 along the axons of nociceptive afferents [29], suggesting that mediators released during the process of Wallerian degeneration may be able to initiate action potentials along the axons of the uninjured afferents. There also is evidence for a redistribution of receptors following injury facilitating afferent activation. For example, evidence from a radiculopathy model suggests that there is not only the recruitment and activation of immune cells within the affected ganglia, but the emergence of functional receptors capable of initiating activity from within the ganglion [30]. Sympathetic postganglionic neurons (SPGNs) also are able to drive activity in primary afferents following nerve injury. This appears to involve the emergence of SPGN–primary afferent coupling in the periphery, within neuromas and within ganglia as well as changes in the pattern of α-adrenergic receptor expression in primary afferents [1]. It is still a matter of debate as to whether SPGN-mediated activation of primary afferents is direct or indirect, in which direct activation may involve P2X receptors and/or a yet to be identified ion channel coupled to α-adrenergic receptors, and indirect activation may involve the release of prostaglandins.
Sensitization
Following tissue injury, afferents become more excitable. This reflects a number of processes, including changes in the density, distribution, and/or biophysical properties of transducers and ion channels critical for spike initiation. For example, nerve injury results in an increase in TRPV1 expression in a subpopulation of uninjured myelinated afferents [31]. TRPV1 is sensitized by mediators, such as NGF [20••], that are increased around uninjured afferents as described above. Because sensitization of TRPV1 is capable of shifting the thermal activation threshold for the channel to within the range of normal body temperature, investigators have suggested that this channel could become spontaneously active and therefore become an important cause for ongoing pain. Although compelling, the absence of heat hyperalgesia in most neuropathic pain patients would argue against this mechanism. Changes in other ion channels that should result in an increase in excitability include a decrease in voltage-gated K+ channels and a shift from Nav1.8 (a high-threshold VGSC underlying spike initiation in the absence of injury) to low-threshold channels such as Nav1.7 [1]. If the increase in excitability is sufficient, normal physiological processes, such as the increase in blood vessel diameter associated with each beat of the heart or resting levels of transmitters such as glutamate or ATP, may become sufficient to evoke afferent activity.
Ectopic activity
The emergence of activity initiated at sites other than peripheral terminals as a source of ongoing pain often precludes the use of topical or local therapeutic modalities and is, therefore, a particularly problematic aspect of neuropathic pain. Two major sources of ectopic activity have been identified.
Ectopic sites of transduction
Following nerve injury, the normal pattern of receptor distribution may be disrupted, with functional receptors targeted to sites of damage along an axon and at the cell body [32]. These changes have been described for thermo- and mechanotransducers in addition to the chemotransducers described above. These changes occur rapidly, such that thermo- and mechanosensitivity is detectable within hours of a nerve transection [32]. The emergence of ongoing activity in injured axons is most readily detectable in muscle afferents, suggesting that this subpopulation of afferents may play a particularly important role in ongoing pain [1]. It also should be noted that transducers are not simply inserted into the distal or cut end of the axon, as one might predict, if transducers transported to the periphery were inserted into the membrane as they piled up at the cut end. Rather, transducers become inserted in the membrane at multiple sites, forming “hot spots” along the injured axon [32]. Although ectopic expression of transducers is generally thought to account for the emergence of phenomena such as the Tinel’s sign or movement-evoked activity, within the context of an axon that is more excitable because of alterations in voltage-gated channel density, these transducers may become a source of ongoing pain.
Membrane oscillations
There is evidence for an increase in both the prevalence and magnitude of membrane oscillations in a subpopulation of injured afferents. Evidence suggests that these oscillations serve as a driving force for ongoing activity, and based on a correlation between the emergence of mechanical hypersensitivity and the increase in membrane oscillations, it has been suggested that this aberrant activity underlies both ongoing pain and mechanical hypersensitivity [1]. Pharmacological analysis of these membrane oscillations suggests that the depolarizing phase of the oscillation is due to a TTX-sensitive VGSC, whereas the repolarizing phase is due to a K+ leak current [1]. Based on the unique biophysical properties of Nav1.3 and the dramatic increase in expression of this channel in injured neurons, this channel was a good candidate for the channel underlying membrane oscillations. A more detailed biophysical analysis of the oscillatory behavior, which is increased with membrane depolarization well above −40 mV [33], suggested this channel may not contribute to the oscillatory behavior as originally suspected. Given mixed results with antisense knockdown of the channel [1], its contribution to neuropathic pain remains in question. Another channel with biophysical properties appropriate for oscillatory behavior is Nav1.7, which undergoes a relatively slow transition to an inactivated state and therefore contributes a significant amount of inward current during slow membrane depolarization [7]. Unfortunately, behavioral data with respect to the role of this channel in neuropathic pain are also conflicting, with knockdown of the channel through genetic means arguing against a role for the channel in neuropathic pain [34], and recent pharmacological data with a compound that blocks Nav1.7 over Nav1.5 or Nav1.8, arguing in favor [35]. Although Nav1.8 may also contribute to oscillatory behavior [7], expression of this channel is initially decreased following nerve injury, and it is TTX-resistant. Because membrane oscillations are detected in putative low-threshold afferents, the specific channels underlying this behavior not only remain to be determined, but so do the mechanisms by which activity in a low-threshold afferent is able to produce pain. It also should be noted that the redistribution of VGSC as such occurs in uninjured afferents following partial nerve injury or at neuromas may contribute to membrane instability and ectopic activity in the absence of membrane oscillations [7].
Although they may or may not contribute to oscillatory behavior, there is evidence that other channels may contribute to the emergence of ongoing or spontaneous activity. One of the most likely of these is a hyperpolarization–activated cyclic nucleotide–modulated (HCN) channel. HCN channels are nonselective cation channels that, when activated, push the membrane potential toward the action potential threshold. The biophysical properties of these channels are such that they may play particularly important roles in driving bursts of activity such as those associated with paroxysms of pain. Pharmacological blockade of these channels eliminates ongoing activity in injured axons in traumatic nerve injury model of neuropathic pain [36]. Interestingly, while both mRNA and protein for HCN1 are reduced in injured neurons, currents are significantly increased [36]. Evidence that this channel also accumulates at the cut end of injured axons raises the possibility that there is an increase in the transport of both mRNA and protein out of the cell body following nerve injury [37]. Minimally, the functional data provide compelling evidence in support of the development of HCN channel blockers for the treatment of neuropathic pain [36,37].
Local signaling within the central terminals
Following tissue injury, there is an increase in the generation of action potentials in the central terminals of nociceptive afferents that propagate back out to the periphery, which is referred to as a dorsal root reflex [38]. This phenomenon suggests that there are not only voltage-gated channels necessary for action potential initiation and propagation in the central terminal of nociceptive afferents, but there are function chemotransducers as well. There also is evidence that both transducer activation and the electrical activity within the central terminals of nociceptive afferents contribute to transmitter release centrally [20••]. Thus, it is reasonable to speculate that there is an increase in local signaling within the dorsal horn following nerve injury, such that activity–independent transmitter release (ie, in the absence of action potentials propagated centrally) may contribute to ongoing pain following nerve injury. Recent data with TRPV1 antagonists suggest that other transducers may contribute to this process. Only compounds with blood-brain barrier permeability had efficacy in the attenuation of mechanical hypersensitivity, suggesting these channels are present and functional on central terminals [39]. The importance of this mechanism may contribute to the therapeutic efficacy of Ca2+ channel blockers, such as gabapentin and SNX-111, for the treatment of neuropathic pain.
The release of transmitter centrally may be facilitated by nerve injury–induced changes in VGCCs. Although reductions in whole cell Ca2+ currents have been described in isolated dorsal root ganglion (DRG) neurons from nerve-injured animals, this may reflect an increase in channel trafficking to central terminals [1]. Consistent with this suggestion, nerve injury is associated with a dramatic increase in the α2δ1 subunit, which is responsible for the trafficking of VGCC [40••]. Furthermore, in mice that lack N-type Ca2+ channels, there is a reduction in neuropathic pain behavior [41]. Given evidence that gabapentin and pregabalin bind to the α2δ subunit [40••] and that an increase in subunit expression is necessary to enable gabapentin-induced block of VGCC [42] and/or reductions in current density [40••], altered trafficking in the presence of nerve injury may contribute to both nerve injury–induced pain and the therapeutic efficacy of the α2δ subunit–binding drugs.
Mechanical allodynia
The combination of detailed quantitative sensory testing and microneurography following peripheral capsaicin injection provides compelling evidence for the following model of mechanical allodynia: activity in capsaicin-sensitive, presumably “mechanically insensitive,” C fibers drives changes within the dorsal horn such that activity in low-threshold afferents that normally signal tactile information are able to signal pain [4]. That similar mechanisms may contribute to mechanical allodynia observed following nerve injury is suggested by electrophysiological data in preclinical models [43]. Given evidence for the emergence of ongoing activity in uninjured C fibers following partial nerve injury, the ability to block this ongoing activity in uninjured C fibers may enable the elimination of ongoing pain in addition to mechanical allodynia [1]. That said, there is still evidence to suggest that changes in the injured fibers may be sufficient to drive changes within the CNS.
Increased expression of putative mechanotransducers
It is also possible that nociceptive afferents are so sensitized following nerve injury that they begin to respond to innocuous mechanical stimuli. An increase in putative mechanotransducers may contribute to this process, and, consistent with this suggestion, there is evidence for an increase in TRPA1, TRPV4, and ASICs. That is, following sciatic nerve ligation, there is an increase in TRPA1 immunostaining and an increase in the percentage of responders and magnitude of response to the potent TRPA1 agonist, mustard oil [44]. The data are not as compelling for TRPV4. However, in one study examining the effects of paclitaxel-mediated toxic neuropathy, TRPV4 knockdown reduced mechanical hypersensitivity [45]. Finally, complex changes in ASIC subunit expression have been described in response to nerve injury. After spared nerve injury, there is a decrease in transcript levels of ASIC1a and ASIC1b, and following sciatic nerve ligation there was a decrease in ASIC1a, ASIC2a, and ASIC3 in injured afferents, whereas ASIC1b was increased in the uninjured population [46]. There also is evidence of an increase in the percentage of ASIC3 expressing neurons in uninjured afferents following nerve injury [47]. These data suggest that changes in ASICs may play an important role in neuropathic pain, at least in the uninjured population.
Evidence also suggests that TRPV1 may contribute to the emergence of mechanical sensitivity following nerve injury. For example, antisense oligonucleotide–induced knockdown of TRPV1 reduced mechanical hypersensitivity following nerve injury [48]. However, the fact that only spinal administration of the TRPV1 antagonist thioxo-BCTC [N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide] attenuated nerve injury–induced mechanical hypersensitivity [48] and that comparable results with systemic administration are only obtained with blood-brain barrier permeant blockers of TRPV1 [39] suggests that this channel only contributes to mechanical sensitivity through an action on the central terminals of nociceptive afferents.
The emergence of novel signaling mechanisms
Evidence suggests that epithelial cells contribute to sensory transduction, particularly in the presence of tissue injury [15]. Thus, it is possible that mechanical allodynia reflects a combination of changes in epithelial cells and primary afferents. There is growing evidence of the former with injury-induced changes in the distribution of several ion channels [49••] as well as the excitability of epithelial cells [50]. Data on the primary afferent side are less consistent with reports of increases [51], decreases [52], and no change [53] in the percentage of cells expressing P2×3 receptors or mRNA levels following nerve injury. These differences likely highlight the fact that type, severity, and timing of traumatic nerve injury, as well as the percentages of injured versus uninjured neurons sampled, are important determining factors for identifying putative mechanisms involved in neuropathic pain. Consistent with an increase in epithelial cell–based signaling, there is evidence of an increase in P2X3 receptors in the uninjured afferent following nerve injury, in contrast to the decrease observed in injured fibers [54]. There also is evidence of an increase in the trafficking of P2×3 receptors to the afferent membrane [53]. Importantly, nonselective P2X-receptor antagonists reduced nerve injury–induced mechanical hypersensitivity [53]. Furthermore, the selective P2X3 and P2X2/3 antagonist, A-317491, reduced nociceptive behavior in an animal model of neuropathic pain, although these effects may be mediated by centrally located purinergic receptors [55]. Similarly, down-regulation of P2X3 receptors with antisense oligonucleotides produced a reduction in mechanical hypersensitivity following nerve ligation [56].
Cold allodynia
The study of isolated sensory neurons in vitro revealed two populations of cold sensitive neurons, one with a low threshold for activation and one with a high-threshold activation consistent with the presence of populations enabling the detection of cool and noxious cold stimuli, respectively [57]. When the spinal nerve ligation model was used to assess changes in both injured and uninjured neurons following nerve injury, a significant increase in the percentage of neurons responsive to both cooling and cold was detected in uninjured, but not injured, neurons [57]. These data were the first evidence that cold allodynia may reflect changes in the primary afferent with an increase in the total amount of afferent input to the CNS with the application of a cold stimulus. Subsequent data not only confirmed that TRPM8 is a cool receptor, but that following nerve injury, there is an increase in the percentage of TRPM8-expressing neurons and enhancement of menthol-evoked currents. These data suggest that TRPM8 up-regulation may contribute to cold hypersensitivity following nerve injury [58]. Cold sensitivity also may reflect the closing of a slowly inactivating 4-aminopyridine sensitive voltage-gated K+ channel [59]. Given the evidence of a nerve injury–induced decrease in a slowly inactivating K+ channel [1], it is also possible that this change contributes to the manifestation of cold allodynia. Minimally, these results suggest that a TRPM8 blocker may effectively attenuate cold allodynia.
Heat hyperalgesia
Nerve injury appears to produce distinct changes in TRPV1 expression in models of both trigeminal and spinal nerve injury that might contribute to injury-induced heat hypersensitivity. Following injury to the inferior alveolar, mental, and lingual nerves, there is an increase in TRPV1 expression in uninjured, but not injured, trigeminal ganglion neurons [60,61]. Comparable results have been obtained following spinal nerve injury [1]. This pattern of changes would be consistent with heat hyper-sensitivity reflecting in intrinsic change in the afferents responsive to thermal stimuli, rather than a change in CNS processing. However, there is debate about the role of TRPV1 and changes in TRPV1 expression in mediating thermal hypersensitivity following nerve injury. For example, there is a correlation between TRPV1 density in skin and mRNA levels in DRG and the magnitude of hyper- and hyposensitivity to heat stimulation, and all changes are abolished in TRPV1 knockout mice [62]. However, TRPV1 null mice did not differ from wild-type mice following partial sciatic nerve ligation with respect to the development of heat hypersensitivity [17].
Conclusions
It is not surprising that neuropathic pain remains difficult to treat given the complexity of the changes initiated in the peripheral nervous system following peripheral nerve injury—a complexity compounded by changes within the CNS and/or changes in the relative impact of factors such as type of injury, site of injury, and time after injury. However, the wealth of data emerging from the study of ion channels that may contribute to neuropathic pain suggest some clear directions for the future. First, it is likely that it will be necessary to develop the diagnostic criteria that will enable identification of the most appropriate target for a therapeutic intervention. Second, because ongoing pain remains the most common and troubling aspect of neuropathic pain, measures of ongoing nociceptive input must become a central component of preclinical studies. Third, although it may not be possible to completely eliminate neuropathic pain, it may be possible to eliminate several positive signs, such as mechanical and cold allodynia, through the targeting of specific ion channels. Finally, through increased understanding of mechanisms controlling ion channel expression and trafficking, particularly those channels selectively expressed in primary afferent neurons, it may ultimately be possible to effectively treat all types of neuropathic pain in the absence of deleterious side effects.
Acknowledgments
Data described in this manuscript were supported in part by National Institutes of Health grants NS059153 (AMH) and NS41384 (MSG).
Footnotes
Disclosure
Dr. Michael S. Gold has a small contract with Traxion Therapeutics and is on the scientific advisory board for TechCommCorp.
No other potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Gold MS, Chessell I, Devor M, et al. Peripheral nervous system targets: Rapporteur report. In: Campbell JN, Basbaum AI, Dray A, et al., editors. Emerging Strategies for the Treatment of Neuropathic Pain. Seattle: IASP Press; 2006. pp. 3–36. [Google Scholar]
- 2.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Dubner R. The neurobiology of persistent pain and its clinical implications. Suppl Clin Neurophysiol. 2004;57:3–7. doi: 10.1016/s1567-424x(09)70337-x. [DOI] [PubMed] [Google Scholar]
- 4.Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol (Lond) 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 6. Attal N, Fermanian C, Fermanian J, et al. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138:343–353. doi: 10.1016/j.pain.2008.01.006. This article offers a very good comprehensive review on neuropathic pain.
- 7.Amir R, Argoff CE, Bennett GJ, et al. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7 Suppl 3:S1–S29. doi: 10.1016/j.jpain.2006.01.444. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Ringkamp M, Hartke TV, et al. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black JA, Langworthy K, Hinson AW, et al. NGF has opposing effects on Na+ channel III and SNS gene expression in spinal sensory neurons. Neuroreport. 1997;8:2331–2335. doi: 10.1097/00001756-199707070-00046. [DOI] [PubMed] [Google Scholar]
- 10.Fjell J, Cummins TR, Dib-Hajj SD, et al. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res. 1999;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard RD, Martinez JJ, Burdick JA, Winkelstein BA. Controlled release of GDNF reduces nerve root-mediated behavioral hypersensitivity. J Orthop Res. 2009;27:120–127. doi: 10.1002/jor.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold MS. Ion channels: recent advances and clinical applications. In: Flor H, Kaslo E, Dostrovsky JO, editors. Proceedings of the 11th World Congress on Pain. Seattle: IASP Press; 2006. pp. 73–92. [Google Scholar]
- 13.LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, et al. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine. 2005;30:1821–1827. doi: 10.1097/01.brs.0000174122.63291.38. [DOI] [PubMed] [Google Scholar]
- 14.Rigaud M, Gemes G, Barabas ME, et al. Species and strain differences in rodent sciatic nerve anatomy: implications for studies of neuropathic pain. Pain. 2008;136:188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289:F489–F495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- 16.Montell C, Caterina MJ. Thermoregulation: channels that are cool to the core. Curr Biol. 2007;17:R885–R887. doi: 10.1016/j.cub.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 18.Karashima Y, Talavera K, Everaerts W, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stucky CL, Dubin AE, Jeske NA, et al. Roles of transient receptor potential channels in pain. Brain Res. 2009 doi: 10.1016/j.brainresrev.2008.12.018. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gold MS, Caterina MJ. Molecular biology of nociceptor transduction. In: Basbaum AI, Bushnell MC, editors. The Senses: A Comprehensive Reference. San Diego: Academic Press; 2008. pp. 43–74. This article offers a very good comprehensive review of the molecular mechanisms underlying primary afferent function.
- 21.Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005;567:53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brierley SM, Carter R, Jones W, 3rd, et al. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price MP, McIlwrath SL, Xie J, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 24.Gold MS. Molecular basis of receptors. In: Merskey H, Loeser JD, Dubner R, editors. The Paths of Pain. Seattle: IASP Press; 2005. pp. 1975–2005.pp. 49–67. [Google Scholar]
- 25.Zimmermann K, Leffler A, Babes A, et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- 26. Dib-Hajj SD, Binshtok AM, Cummins TR, et al. Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment. Brain Res Rev. 2008 Dec 25; doi: 10.1016/j.brainresrev.2008.12.005. (Epub ahead of print) The authors of this article provide a comprehensive review on voltage-gated sodium channels in pain states.
- 27.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 28.Jevtovic-Todorovic V, Todorovic SM. The role of peripheral T-type calcium channels in pain transmission. Cell Calcium. 2006;40:197–203. doi: 10.1016/j.ceca.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Fischer MJ, Reeh PW. Sensitization to heat through G-protein-coupled receptor pathways in the isolated sciatic mouse nerve. Eur J Neurosci. 2007;25:3570–3575. doi: 10.1111/j.1460-9568.2007.05582.x. [DOI] [PubMed] [Google Scholar]
- 30.Ma C, Greenquist KW, Lamotte RH. inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- 31.Rashid MH, Inoue M, Kondo S, et al. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J Pharmacol Exp Ther. 2003;304:940–948. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- 32.Grossmann L, Gorodetskaya N, Teliban A, et al. Cutaneous afferent C-fibers regenerating along the distal nerve stump after crush lesion show two types of cold sensitivity. Eur J Pain. 2008 Oct 29; doi: 10.1016/j.ejpain.2008.09.004. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Liu CN, Devor M, Waxman SG, Kocsis JD. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol. 2002;87:2009–2017. doi: 10.1152/jn.00705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassar MA, Levato A, Stirling LC, Wood JN. Neuropathic pain develops normally in mice lacking both Nav1.7 and Nav1.8. Mol Pain. 2005;1:24. doi: 10.1186/1744-8069-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyt SB, London C, Gorin D, et al. Discovery of a novel class of benzazepinone Na(v)1.7 blockers: potential treatments for neuropathic pain. Bioorg Med Chem Lett. 2007;17:4630–4634. doi: 10.1016/j.bmcl.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 36.Chaplan SR, Guo HQ, Lee DH, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang YQ, Xing GG, Wang SL, et al. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain. 2008;137:495–506. doi: 10.1016/j.pain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Willis WD., Jr Dorsal root potentials and dorsal root refl exes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 39.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2008 Dec 25; doi: 10.1016/j.brainresrev.2008.12.006. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 40. Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin: calcium channel alpha(2)-delta [Ca(v)alpha(2)-delta] ligands. Pain. 2009 Jan 6; doi: 10.1016/j.pain.2008.11.019. (Epub ahead of print) This comprehensive review focuses on molecular mechanisms underlying the actions of gabapentin and pregabalin.
- 41.Saegusa H, Kurihara T, Zong S, et al. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20:2349–2356. doi: 10.1093/emboj/20.10.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li CY, Zhang XL, Matthews EA, et al. Calcium channel alpha(2)delta(1) subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoffnegger D, Ruscheweyh R, Sandkuhler J. Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain. 2008;135:300–310. doi: 10.1016/j.pain.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Ji G, Zhou S, Carlton SM. Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience. 2008;154:1054–1066. doi: 10.1016/j.neuroscience.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant AD, Cottrell GS, Amadesi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omori M, Yokoyama M, Matsuoka Y, et al. Effects of selective spinal nerve ligation on acetic acid-induced nociceptive responses and ASIC3 immunoreactivity in the rat dorsal root ganglion. Brain Res. 2008;1219:26–31. doi: 10.1016/j.brainres.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 48.Christoph T, Gillen C, Mika J, et al. Antinociceptive effect of antisense oligonucleotides against the vanilloid receptor VR1/TRPV1. Neurochem Int. 2007;50:281–290. doi: 10.1016/j.neuint.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 49. Dussor GO, Koerber HR, Oaklander AL, et al. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res. 2009 doi: 10.1016/j.brainresrev.2008.12.013. (in press) In this comprehensive review, the authors focus on nucleotide signaling and its relevance to chronic pain.
- 50.Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep. 2006;7:440–446. doi: 10.1007/s11934-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 51.Shinoda M, Kawashima K, Ozaki N, et al. P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain. 2007;8:588–597. doi: 10.1016/j.jpain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Wang R, Guo W, Ossipov MH, et al. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Li GW, Wang C, et al. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Tsuzuki K, Kondo E, Fukuoka T, et al. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- 55.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Honore P, Kage K, Mikusa J, et al. Analgesic profile of intrathecal P2X(3) antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain. 2002;99:11–19. doi: 10.1016/s0304-3959(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 57.Djouhri L, Wrigley D, Thut PD, Gold MS. Spinal nerve injury increases the percentage of cold-responsive DRG neurons. Neuroreport. 2004;15:457–460. doi: 10.1097/00001756-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 58.Xing H, Chen M, Ling J, et al. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–13690. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viana F, de la Pena E, Belmonte C. Specificity of cold thermo transduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- 60.Biggs JE, Yates JM, Loescher AR, et al. Changes in vanilloid receptor 1 (TRPV1) expression following lingual nerve injury. Eur J Pain. 2007;11:192–201. doi: 10.1016/j.ejpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Kim HY, Park CK, Cho IH, et al. Differential changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain. 2008;9:280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Pabbidi RM, Yu SQ, Peng S, et al. influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain. 2008;4:9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]