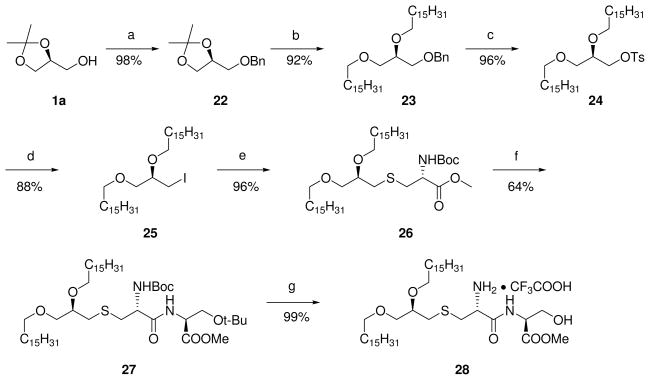
Scheme 4.
Reagents and conditions: a: BnBr, NaH, DMF, 0 °C- r.t., 8 h;
b: i) 70% AcOH, r.t., 12 h; ii) C16H33I, NaH, DMF, 0 °C- r.t., 8 h;
c: i) Pd/C, H2, 8 h; ii) TsCl, pyr, DMAP, CH3CN, 70 °C, 12 h;
d: I2, KI, DMF, 80 °C, 24 h;
e: Boc-L-Cys-OMe, TEA, DMF, 85 °C, 2 h;
f: i) LiOH, THF/H2O, r.t., 10 h; ii) H-L-Ser(tBu)-OMe •HCl, EDCI, EDIPA, DMAP, DCM, 0 °C-r.t., 8 h;
g: TFA, r.t., 30 min.