Abstract
Purpose
To determine the neurofunctional basis of verbal memory dysfunction in women with metastatic breast cancer. This objective was based on previous research suggesting memory and other cognitive deficits in this population. We attempted to determine if verbal memory impairments were related to the most commonly studied disease parameters including adjuvant chemotherapy and chronic stress-related disruption of limbic system structures.
Experimental Design
We utilized functional magnetic resonance imaging (fMRI) to test our hypothesis that women with breast cancer would demonstrate significantly lower brain activation during a verbal declarative memory tasks compared to age and education-matched healthy female controls. We also assessed several stress-related variables including diurnal cortisol levels to test our hypothesis that women with breast cancer would demonstrate higher stress and this would contribute to brain activation deficits during memory tasks.
Results
Women with breast cancer had significantly lower prefrontal cortex activation during the memory encoding condition compared to controls. However, the breast cancer group demonstrated significantly greater activation than controls during the recall condition in multiple, diffuse brain regions. There were no significant differences between the groups in stress-related variables. Women who were treated with CMF chemotherapy demonstrated lower prefrontal cortex activation during memory encoding.
Conclusions
These results suggest that women with metastatic breast cancer may be at risk for verbal memory impairments as a result of altered functional brain activation profiles. These findings may be associated with chemotherapy type and/or other aspects of the breast cancer disease process.
INTRODUCTION
Women with breast cancer (BC) may have an increased risk for long-term cognitive-behavioral impairments including those of executive function and memory, likely due to neurotoxic side effects of chemotherapy (1–3). The incidence of these impairments is uncertain but has been reported to range from 28–75% among breast cancer patients receiving CMF (4). Cognitive-behavioral impairments significantly extend disease-related disability, affecting home, educational and occupational activities. Additionally, the high prevalence of BC and increasing survival rates contribute to a large and growing cohort of cognitively affected individuals (3). Currently, there are no specific treatments for these cognitive impairments and no preventive interventions are available.
Neuroimaging studies provide insight regarding the neurobiologic mechanisms underlying cognitive impairment in various populations. To date, there have been only four such studies conducted in BC to our knowledge. These reports indicate altered cerebral metabolism and decreased volume in executive function regions, including prefrontal cortex, basal ganglia and the cingulate gyrus in women with BC compared to controls (5, 6). Additionally, reduced corpus callosum genu white matter pathway integrity (i.e. fractional anisotropy) was noted in a small sample of women with BC compared to controls and was correlated with lower processing speed (7). A case study of monozygotic twins discordant for BC indicated increased white matter hyperintensities and altered frontal-parietal functional brain activation in the twin with BC (8). This case study represents the only functional MRI (fMRI) study conducted to date. FMRI allows in vivo assessment of brain function by detecting blood flow differences and is widely used to study brain-behavior relationships in children and adults (9–12). Group fMRI studies could significantly advance our understanding regarding the neurobiologic mechanisms underlying cognitive deficits in women with BC. Additionally, fMRI studies are being increasingly utilized to guide and provide potential targets for cognitive interventions (13). This study aimed to implement an fMRI paradigm to investigate verbal memory function in women with BC.
Some studies suggest that cognitive deficits in women with BC are associated, at least in part, with illness-related stress (3, 14). Abnormal diurnal cortisol patterns have been observed among women with breast cancer (15, 16) and cortisol is known to play a vital role in cognitive and emotional function. In the present study, we obtained both psychological and physiological measures of stress including self-rating questionnaires, vital statistics and salivary cortisol to use as covariates of nuisance in our analyses of brain activation.
Many studies of cognitive outcome in women with BC do not include chemotherapy in the analyses due to the significant variation among patients in terms of chemotherapy regimen. However, cognitive deficits in survivors of BC have been associated with cytotoxic chemotherapy such as methotrexate (17) and hormonal blockade with tamoxifen (5, 18–20). Animal studies have suggested that certain chemotherapeutic agents including methotrexate, carmustine, cisplatin, 5-fluorouracil, and cytarabine may be more toxic to white matter progenitor and hippocampal stem cells than they are to actual cancer cells (21–23). We collected chemotherapy and tamoxifen treatment information in order to conduct exploratory analyses regarding the effects of particular treatment variables on brain activation during verbal memory.
METHODS
Participants
Fourteen women (mean age = 55.1 ± 8.0 years, range = 43–65) with metastatic (N = 8) or locally advanced (N = 6) breast cancer and 14 age and education-matched healthy female controls (mean age = 54.2 ± 8.0, range = 40–65, p = .78; mean education rank = 5 ± 2; p = .83) participated in this study. Education data were gathered using the following ranking system: 1 = Less than High School, 2 = Graduated from High School/GED, 3 = Completed Trade School, 4 = Some College, 5 = Bachelor’s Degree, 6 = Some Graduate School, 7 = Master’s Degree, 8 = Ph.D., M.D., and/or J.D.
All women with BC had a history of adjuvant chemotherapy treatment and 11 had a history of radiation therapy to the neck, affected breast, axilla and/or lumbar spine. Chemotherapy types included 6 cycles of cyclophosphamide, methotrexate and 5-fluorouracil (CMF, N = 5) or 4–8 cycles of adriamycin, cyclophosphamide, and taxol/taxotere (ACT, N = 9). All chemotherapy and radiation treatments had to be completed at least 6 months prior to enrollment to be eligible for the study (mean time since last chemotherapy/radiation = 3.3 ± 3.3 years, range = .5 – 10.3). Eleven of the patients also had taken tamoxifen during their course of BC treatment (mean time since tamoxifen = 5.3 ± 2.3 years, range = 2–9). Six of the 11 BC/tamoxifen patients took tamoxifen after chemotherapy/radiation, 3 took it concurrently with chemotherapy/radiation and 2 took tamoxifen before chemotherapy/radiation (these 2 were treated in 1991 and 1995, respectively). Information regarding anti-cancer treatments was obtained using both patient and physician report as well as medical records, when available.
Healthy female controls were recruited via community flyers and internet postings restricted to the same zip codes as the women with BC to reduce differences in socioeconomic status. There were no significant differences between the groups in terms of minority status (X2 =.003, p= .74).
All potential participants were excluded for MRI contraindications (e.g. metallic implants, biomedical devices) and were free from medications that affect cortisol (e.g. hydrocortisone, megestrol) and functional MRI (e.g. antipsychotics, sedatives, MAO inhibitors, tricyclic anti-depressants). Participants with BC were excluded for active cancers within the past 10 years other than breast cancer, basal cell or squamous cell carcinomas of the skin, in situ cancer of the cervix, positive supraclavicular lymph nodes as the only metastatic lesion at the time of initial diagnosis, history of neurologic conditions including metastases to the brain as well as history of learning or other developmental disability, preterm birth and psychiatric or chronic medical conditions not related to BC. Healthy controls were excluded for any history of learning or other developmental disability, preterm birth, psychiatric, neurologic or chronic medical condition. Their participation in the present study was approved by the Stanford University Institutional Review Board and all participants gave written informed consent before beginning the study.
Distress Measures
Cortisol Collection
As salivary cortisol has been found to be a reliable tool for investigations of HPA activity (24–26), saliva samples were collected on two consecutive baseline days, not including the day of the MRI. Samples were collected at awakening, 12PM, 5PM and 9PM on both days using cortisol-specific self-collection kits, according to a previously published protocol (15, 16). Participants were provided with both verbal and written collection instructions. Due to significant variance in the cortisol data, all cortisol variables were log transformed (see Table 1). Cortisol level could not be obtained for 3 women with BC and 2 controls.
Table 1.
Demographic, fMRI behavioral performance and distress measures data
| N | Breast Cancer |
N | Control | t | F | U | p | |
|---|---|---|---|---|---|---|---|---|
| age (years) | 14 | 55.1 (8.0) | 14 | 54.2 (8.0) | .28 | .78 | ||
| education (rank)* | 14 | 5 (2) | 14 | 5 (2) | 87 | .83 | ||
| last chemo/radiation (years) | 14 | 3.3 (3.3) | ||||||
| last tamoxifen (years) | 14 | 5.3 (2.3) | ||||||
| encoding between group ROI | 14 | .21 (.27) | 14 | .47 (.23) | 9.3 | .005 | ||
| encoding % correct | 14 | .91 (.07) | 14 | .90 (.08) | .26 | .79 | ||
| encoding reaction time (milliseconds) |
14 | 1201 (177) | 14 | 1337 (166) | −2.0 | .06 | ||
| recall between group ROI | 14 | .34 (.16) | 14 | .18 (.20) | 4.9 | .04 | ||
| recall % correct | 14 | .82 (.14) | 14 | .76 (1.8) | .88 | .39 | ||
| recall reaction time (milliseconds) |
14 | 1357 (139) | 14 | 1124 (141) | 3.6 | .002 | ||
| baseline cortisol slope** | 11 | −.16 (.06) | 12 | −.15 (.08) | −.44 | .67 | ||
| Cortisol Questionnaire | 11 | 3.3 (.75) | 12 | 3.1 (.73) | .78 | .44 | ||
| average heart rate | 14 | 68.3 (5.4) | 14 | 64.7 (10.3) | 1.0 | .31 | ||
| average respiratory rate | 14 | 19.6 (2.7) | 14 | 21.0 (4.6) | −.86 | .40 | ||
| WAI Distress Composite | 14 | 57 (15) | 14 | 51 (9) | 1.3 | .28 | ||
| WAI Restraint Composite | 14 | 134 (6) | 14 | 130 (9) | .96 | .34 | ||
| WAI Repressive- Defensive/Restraint Composite |
14 | 80 (9) | 14 | 74 (8) | 2.6 | .12 | ||
| Repressive-Defensive Subscale | 14 | 35 (8) | 14 | 31 (6) | 1.9 | .18 | ||
| Anxiety Subscale | 14 | 19 (5) | 14 | 19 (6) | .16 | .69 | ||
| BDI Total Score | 14 | 7 (4) | 14 | 5 (6) | 1.2 | .21 |
Data are shown as mean (standard deviation);
Education ranking key: 1 = Less than High School, 2 = Graduated from High School/GED, 3 = Completed Trade School, 4 = Some College, 5 = Bachelor’s Degree, 6 = Some Graduate School, 7 = Master’s Degree, 8 = Ph.D., M.D., and/or J.D.; ROI = region of interest representing the mean activation intensity value across all voxels in the areas of significant between-group difference; WAI = Weinberger Adjustment Inventory;
log transformed data; BDI = Beck Depression Inventory
Cortisol Questionnaire
is a 12-item self-rating measure designed to assess factors affecting stress and cortisol levels related to physical activity, stress level, sleep and subjective sense of health.
Psychophysiology
Heart and respiratory rate were measured during the entire MRI scan.
Weinberger Adjustment Inventory (WAI)
The WAI is an 84-item self-rating measure of typological stress coping strategy including anxiety, restraint and repression (27). Although the WAI yields multiple subscale and composite scores, we examined only the three composite scores: Repressive-Defensive/Restraint, Distress, and Restraint, as well as the two subscales that we have previously shown to differentiate women with BC and healthy female controls: Anxiety and Repressive-Defensiveness (15). This subset of WAI scores were used in order to reduce the number of comparisons and the amount of measurement error associated with multiple scales and to constrain this analysis to the WAI scales that are most consistent with our a priori hypothesis regarding the effects of stress on memory function.
Beck Depression Inventory (BDI)
The BDI is a 21-item self-rating inventory of depressive symptomology including sadness, suicidal ideation, fatigue, loss of interest and sleep disruption (28).
MRI Acquisition Protocols
We acquired structural scans using a protocol that provides a high level of anatomic resolution and tissue contrast: 3D IR prepared FSPGR, TR: minimum, TE: minimum, flip: 15 degrees, TI: 300 ms, BW: +/−31.25 kHz, FOV: 22cm, Phase FOV: 0.80 FOV (22 × 15.4), slice thickness: 1.5mm, 128 slices (to get 124 slice locations), 256×256 @ 3 NEX.
Acquisition of functional MRI (fMRI) data was based on a spiral-in/out blood oxygenation level dependent (BOLD) protocol (29). This provides for improved BOLD signal in regions of interest such as the amygdala and hippocampus. Thirty-two axial slices (3 mm thick, 1 mm skip) parallel to the AC–PC line and covering the whole brain were imaged with a temporal resolution of 2 seconds using a T2* weighted gradient echo spiral pulse sequence (TR = 2000 msec, TE = 30msec, flip angle = 80° and 1 interleave). The field of view was 200 × 200 mm2, and the matrix size as 64 × 64, giving an in-plane spatial resolution of 3.125mm. An automated high-order shimming method based on spiral acquisitions was employed to reduce field heterogeneity.
Functional MRI Tasks
Stimulus Presentation
All stimuli were presented using E-Prime software (Psychology Software Tools, Pittsburgh, PA), which also triggered the initiation of the scan. Stimuli were presented using a custom-adapted system that projects stimuli at high resolution (1024 × 768) onto a screen attached to the head coil. Subjects look directly upward at a mirror to view the stimuli. Behavioral responses were recorded using a 4-button fiber optic finger switch system. Task performance was acquired simultaneously while subjects performed the tasks in the scanner.
Verbal Declarative Memory Encoding
The encoding condition of the fMRI task required subjects to view visually presented nouns and make a semantic discrimination for each by pressing button 1 if the word represented something man-made and button 2 if the word represented something not man-made.
Verbal Declarative Memory Recall
During the recall task condition, subjects were again presented with single nouns and were asked to press button 1 if they recognized the word from the previous task and button 2 if they did not.
The specific details of these tasks are described elsewhere (30).
fMRI Preprocessing
Using Statistical Parametric Mapping 2 (SPM2) software1 images were realigned to correct for head movement using least square minimization, normalized to a standardized template in order to directly compare brain activation across subjects, stimulus types and experimental conditions; and smoothed to reduce the effects of noise. Images for each subject were then visually assessed for correct spatial normalization using in-house software that creates 3D renders and slice maps (axial, coronal, sagittal) of raw, normalized and statistical images for comparison with template examples of correctly aligned images. We also employed our automated in-house software program, “ArtRepair” (http://spnl.stanford.edu/tools/ArtRepair/ArtRepair.htm) to automatically detect and repair motion and signal intensity outliers as well as other artifacts at the volume, slice and voxel level (31).
Statistical Analyses
Distress Measures
Group differences in baseline 2-day cortisol slope, stress questionnaire, heart rate and respiration rate were conducted using independent t-tests and WAI scores were measured using MANOVA in SPSS 16.0. As there were no between group differences in distress measures, we did not include them as covariates in further analyses.
fMRI Task Performance Data
fMRI task performance data (percent correct, reaction time) were compared between groups using independent t-tests.
fMRI Whole Brain Activation Data
Statistical analyses were performed SPM2 (32). Any images that represented movement, signal and/or rotation outliers as indicated by the ArtDetect5 procedure were excluded from the statistical analyses. The number of outlier images excluded did not total greater than 1% of the total number of images for any scan.
A within-subjects procedure was used to model all the effects of interest for each participant. Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low-frequency noise was removed with a high-pass filter (0.5 cycles/min) applied to the fMRI time series at each voxel. A temporal smoothing function (Gaussian kernel corresponding to dispersion of 8 sec) was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. For each participant, brain activation related to each fMRI task was determined by contrasting experimental and control conditions.
Group analysis was performed using a random-effects model that incorporated a two-stage hierarchical procedure. This model estimates the error variance for each condition of interest across participants, rather than across scans (33) and therefore provides a stronger generalization to the population from which data are acquired. Individual contrast images were analyzed using a general linear model to determine voxel-wise t-statistics. One contrast image was generated per participant, per effect of interest. A one-way t-test was then used to determine whole brain within-group activation for each task. Between group differences in brain activation for each task was determined using an analysis of variance model masked with the within-group SPM map to restrict these analyses to the regions of significant within-group brain activation and further reduce the number of statistical tests performed. The statistics for all analyses were normalized to Z scores, and significant clusters of activation were determined using height and extent thresholds of p < 0.05, controlling for multiple comparisons using false discovery rate. Activation foci were superimposed on high-resolution T1-weighted images and their locations interpreted using known neuroanatomical landmarks.
The MarsBaR2 region of interest (ROI) toolbox in SPM2 was used to create ROIs representing the regions of significant between group differences in activation. This is accomplished by creating a mask of the significant activation clusters from the between group contrasts. The ROI Toolbox3 in SPM2 was then used to extract the mean intensity value across all voxels in the ROIs for each subject. These ROI values were then used in SPSS 16 as dependent variables in general linear models (GLM) with group as a fixed factor and age as a covariate to confirm the whole brain between group SPM results while removing the effects of age. ROI activation was correlated (two-tailed Pearson) with task response data that were significantly different between the groups to explore the associations between brain activation and task performance differences.
For the BC group only, ROI values were also entered as dependent variables in a multiple regression with tamoxifen (1 = yes, 0 = no) and chemotherapy type (0 = ACT, 1 = CMF) as independent variables. ROI values were correlated (two-tailed Pearson) with time since the last chemotherapy/radiation treatment. These analyses were conducted to explore the effect of treatments on brain activation in the regions of between group difference.
RESULTS
Distress Measures
There were no between group differences in any of the distress-related variables (Table 1).
Memory Encoding fMRI
The two groups performed the encoding condition similarly in terms of accuracy (p = .79) but there was a nonsignificant trend for the women with BC to show faster reaction times (p = .06). The BC group demonstrated significant brain activation in bilateral inferior occipital gyrus extending into the left fusiform gyrus, left cerebellum and right middle occipital gyrus and the left inferior frontal gyrus extending into the left insula (Table 2). The control group demonstrated significant activation in the left superior frontal gyrus extending into bilateral anterior cingulate, right superior frontal, bilateral middle frontal, left inferior frontal and bilateral superior temporal gyri as well as bilateral basal ganglia (Table 2).
Table 2.
Within and between group results of fMRI analyses indicating significant brain activation during the encoding task (experimental minus control contrast).
| P value (FDR corrected) |
cluster size |
T score |
MNI Coordinates |
Location Description |
|---|---|---|---|---|
| Breast Cancer Group | ||||
| .04 | 1048 | 7.33 | −44, −86, −12 | left inferior occipital gyrus extending into left fusiform gyrus and left cerebellum |
| .04 | 1535 | 6.65 | −40, 28, 12 | left inferior frontal gyrus extending into left insula |
| .05 | 541 | 6.34 | 36, −94, −8 | right inferior occipital gyrus extending into right middle occipital gyrus |
| Control Group | ||||
| .003 | 50983 | 10.25 | −8, 6, 52 | left superior frontal gyrus extending into bilateral anterior cingulate, right superior frontal, bilateral middle frontal, left inferior frontal and bilateral superior temporal gyri and bilateral basal ganglia |
| Control > Breast Cancer | ||||
| .01 | 6448 | 3.92 | −14, −8, 78 | left superior frontal gyrus extending into the right superior frontal gyrus, bilateral middle frontal gyri and left postcentral gyrus |
| Breast Cancer > Control = none | ||||
Height and extent threshold: p < .05, FDR
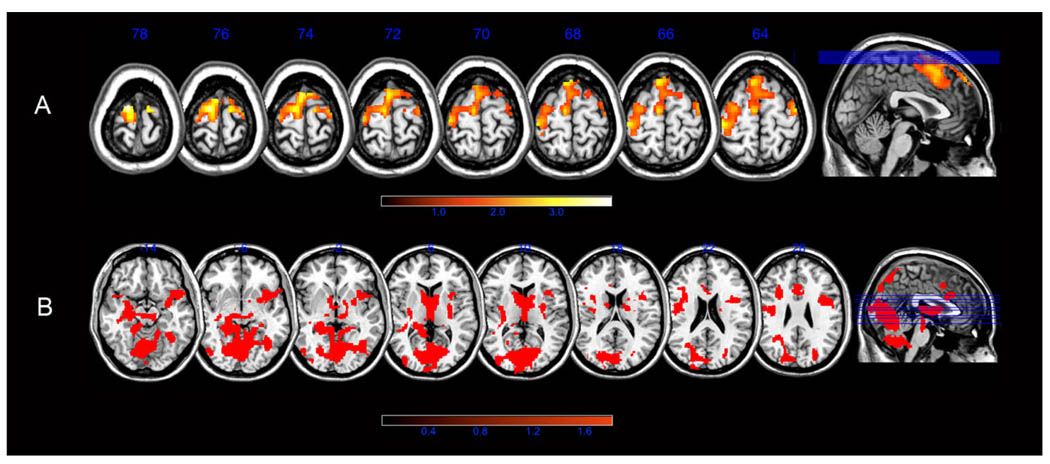
The control group demonstrated significantly greater activation in the left superior frontal gyrus extending into the right superior frontal gyrus, bilateral middle frontal gyri and left postcentral gyrus, compared to the BC group (Table 2, Figure 1a). The BC group showed no regions of significantly greater activation compared to controls. These between group results were irrespective of age, confirmed by ROI GLM analysis (F = 9.25, p = .005, Table 1).
Figure 1.
(A) Axial SPM maps showing regions of significantly greater functional brain activation in controls compared to women with BC during memory encoding. (B) Regions of significantly increased brain activation in women with BC compared to controls during memory recall. Color bar shows T score range. Slice labels indicate MNI z coordinate.
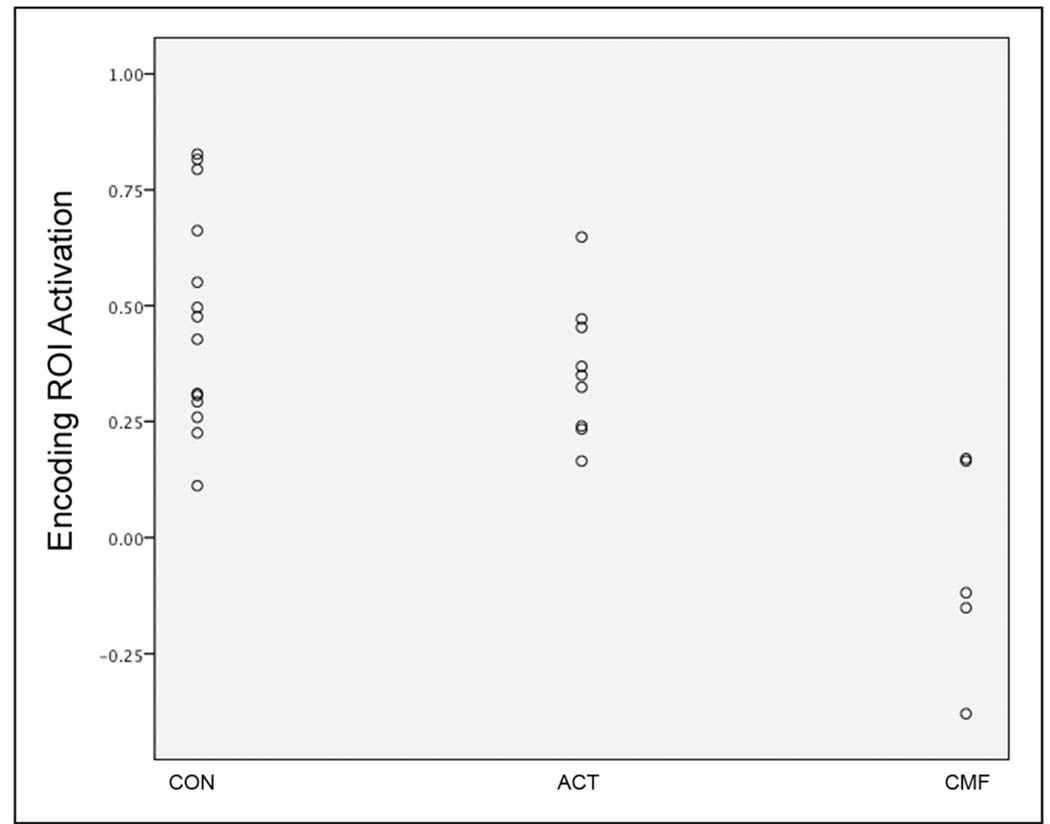
Chemotherapy type, but not tamoxifen, predicted activation in the between group difference ROI (i.e. the regions illustrated in Figure 1a; adjusted R2 = .52, F = 7.4, p = .01) in the BC group. Given the negative coefficient values for chemotherapy type (B = −.40, t = −3.2, p = .009), women with BC who received CMF chemotherapy demonstrated significantly lower activation in the ROI. A post hoc GLM examining encoding ROI activation between controls, CMF and ACT groups indicated significant differences between CMF and controls (p < .0001) as well as CMF and ACT (p = .001) but no significant difference between controls and ACT (p = .25). This finding is illustrated graphically in Figure 2. There were no significant differences between ACT and CMF groups in terms of age, ethnicity, education, stress variables, time since tamoxifen or fMRI task performance. Time since chemotherapy/radiation treatment was significantly greater in women treated with CMF compared to ACT (p = .006). However, the difference in encoding ROI activation between the CMF and ACT groups remained even after covarying for time since chemotherapy/radiation treatment (p < .0001).
Figure 2.
Scatterplot illustrating mean activation in the encoding ROI (i.e. prefrontal cortex) in controls (CON), women with breast cancer treated with ACT and women with breast treated with CMF.
Encoding ROI activation was significantly correlated with reaction time in controls (r = .58, p = .04) but not in the BC group (r = .04, p = .90). The difference in these correlations (Fisher r–z transformation) was nearly significant (z = −1.46, p = .07). Encoding ROI activation in the BC group was not correlated with time since treatment.
Memory Recall fMRI
The two groups demonstrated similar accuracy on the recall task (p = .39) but the BC group showed significantly slower reaction time (p = .002). Women with BC demonstrated significant brain activation in the left inferior occipital gyrus extending into right inferior and bilateral middle and superior occipital gyri, bilateral cerebellum, bilateral cuneus and precuneus, bilateral inferior and superior parietal lobe, bilateral fusiform gyri, bilateral superior and inferior temporal gyri, left middle temporal gyrus, bilateral hippocampus, bilateral dorsolateral prefrontal cortex, bilateral cingulate, bilateral medial frontal gyri, bilateral basal ganglia and bilateral lateral orbital gyri (Table 3). Controls significantly activated bilateral inferior frontal gyri extending into right middle frontal gyrus, bilateral cingulate gyri extending into left superior frontal gyrus, left cuneus extending into left lingual and fusiform gyri, right middle occipital gyrus extending into right cuneus, right inferior parietal lobe extending into right superior parietal lobe and right precuneus, right cerebellum, and left superior parietal lobe (Table 3).
Table 3.
Within and between group results of fMRI analyses indicating significant brain activation during the recall task (experimental minus control contrast).
| P value (FDR corrected) |
cluster size |
T score |
MNI Coordinates |
Location Description |
|---|---|---|---|---|
| Breast Cancer Group | ||||
| <.0001 | 92477 | 15.06 | −38, −84, −12 | left inferior occipital gyrus extending into right inferior and bilateral middle and superior occipital gyri, bilateral cerebellum, bilateral cuneus and precuneus, bilateral inferior and superior parietal lobe, bilateral fusiform gyri, bilateral superior and inferior temporal gyri, left middle temporal gyrus, bilateral hippocampus, bilateral dorsolateral prefrontal cortex, bilateral cingulate, bilateral medial frontal gyri, bilateral basal ganglia and bilateral lateral orbital gyri |
| Control Group | ||||
| .003 | 1188 | 14.33 | 36, 22, −8 | right inferior frontal gyrus |
| .005 | 2152 | 9.19 | −32, 22, −10 | Left inferior frontal gyrus |
| .006 | 1526 | 8.52 | 6, 20, 48 | Right anterior cingulate extending into left anterior cingulate and left superior frontal gyrus |
| .007 | 965 | 8.40 | −26, −94, −4 | left cuneus extending into left lingual and fusiform gyri |
| .009 | 815 | 7.90 | 58, 12, 30 | right inferior frontal gyrus extending into right middle frontal gyrus |
| .01 | 490 | 6.32 | 30, −92, −2 | right middle occipital gyrus extending into right cuneus |
| .01 | 473 | 6.30 | 36, −64, 44 | right inferior parietal lobe extending into right superior parietal lobe and right precuneus |
| .02 | 381 | 5.93 | 36, −70, −30 | right cerebellum |
| .02 | 516 | 4.96 | −32, −64, 42 | right cerebellum, and left superior parietal lobe |
| Breast Cancer > Control | ||||
| <.0001 | 20056 | 5.20 | 46, 10, −14 | right superior temporal gyrus extending into bilateral fusiform, bilateral lingual gyri, left hippocampus, bilateral basal ganglia, right precentral gyrus, right superior and inferior frontal gyri, right middle frontal gyrus, bilateral inferior frontal gyrus, right cingulate gyrus, bilateral insula, bilateral parahippocampal gyrus, bilateral cuneus, bilateral precuneus, bilateral superior parietal lobe and cerebellum |
| Control > Breast Cancer = none | ||||
Height and extent threshold: p < .05, FDR
Compared to controls, the BC group demonstrated significantly greater activation in right superior temporal gyrus extending into bilateral fusiform, bilateral lingual gyri, left hippocampus, bilateral basal ganglia, right precentral gyrus, right superior and inferior frontal gyri, right middle frontal gyrus, bilateral inferior frontal gyrus, right cingulate gyrus, bilateral insula, bilateral parahippocampal gyrus, bilateral cuneus, bilateral precuneus, bilateral superior parietal lobe and cerebellum (Table 3, Figure 1b). Controls did not show any regions of greater activation compared to women with BC during the recall task. These between group results were irrespective of age as confirmed by ROI GLM analysis (F = 4.93, p = .04, Table 1).
Treatment variables were not associated with activation in the between group difference ROI for the recall task in the BC group. Recall ROI activation was not correlated with reaction time in either group.
Women with metastatic disease were significantly older than women with locally advanced breast cancer (t = 2.9, p = .01). However, there were no significant differences between women with metastatic or locally advanced breast cancer in terms of ethnicity, education, stress variables, time since tamoxifen, time since chemotherapy/radiation, fMRI task performance or fMRI ROI activation, even when controlling for age.
DISCUSSION
To our knowledge, this is the first cohort study of functional brain activation in women with breast cancer (BC). We demonstrated reduced prefrontal cortex activation in women with BC compared to controls during the semantic encoding phase of a verbal declarative memory task. During the recall condition of the task, women with BC showed significantly increased spatial extent of cortical activation compared to controls. Regions of increased activation included those less commonly involved in declarative verbal memory retrieval (e.g. orbital prefrontal, right superior temporal gyrus, inferior temporal gyrus, occipital gyri) as indicated by studies utilizing a similar fMRI task (34). These findings suggest overactivation of brain regions during this memory task. These results were irrespective of age and therefore not likely explained by aging or menopause. Women with BC were similar to controls in performance accuracy during both task conditions but showed a tendency to respond more quickly during encoding and significantly more slowly during recall.
Previous studies have demonstrated abnormalities of the prefrontal cortex (5, 6) in women with BC who have undergone adjuvant chemotherapy. The prefrontal cortex is known to play a critical role in declarative memory (35). Our present results offer increased insight into and more specific neural correlates of verbal memory difficulties in certain women with BC. Specifically, during memory encoding, women with BC showed lower prefrontal activation as well as quicker, perhaps more impulsive, response time compared to controls. Reduced prefrontal resources may indicate lower attention and/or organizational skills associated with learning and memory. For example, women treated with CMF chemotherapy for BC may have more difficulty attending to stimuli and/or engaging in organizational or mnemonic strategies for memorization. Increased prefrontal activation was associated with increased reaction time in controls but not in the BC group. It appears that the controls were able to utilize more prefrontal resources and took more time to encode the stimuli. Because the stimuli were not properly encoded, the recall condition required a significant increase in neural effort in the BC group. These findings have implications for potential treatments for verbal memory impairments in women with BC. For example, focusing cognitive interventions on executive, prefrontal skills could provide rehabilitation that will improve encoding efficiency and reduce recall effort.
Cognitive difficulties in chemotherapy-treated BC have been somewhat controversial because neuropsychological testing scores are often within normal limits (2) or not different from controls (18). There may be significant discrepancies between subjective and objective measures of cognitive ability as well as associations between subject cognitive complaints and psychological distress (18) leading some to conclude that treatment-related cognitive deficits in BC are merely based on anxiety about performance. Our results show that, although some women with BC might perform as accurately as controls on certain memory tasks, they have brain-based deficits related to memory encoding and thus require a great deal more neural effort when attempting to recall information. This likely results in increased cognitive fatigue and frustration, resulting in negative subjective evaluation of cognitive ability.
Some studies suggest cognitive deficits associated with BC and its treatment tend to resolve over time, at least in some patients (36–38). However, our sample of women with BC were, on average, 3.3 years post-treatment and we showed no relationship between increased time since treatment and improved brain function during memory tasks. Some women with BC may be able to compensate for neurofunctional changes associated with chemotherapy while others cannot. For example, one study suggested that women with BC who have the E4 variant of the APOE gene, which is believed to affect memory function and elevate risk for Alzheimer’s dementia (39), might be at higher risk for cognitive difficulties than women with BC who carry other APOE alleles (40). Further studies are required to determine if there are other variables that predict cognitive outcome in women with BC.
Contrary to our hypotheses, we did not find that our sample of women with BC was more distressed than controls. It is possible that only those women who do not have significant distress agreed to an MRI, which can be a stressful and intimidating procedure. Sampling bias may thus influence our findings. However, our results show that neurofunctional deficits exist even in seemingly non-distressed women with BC during memory tasks. Therefore verbal memory impairments in some women with BC cannot be explained by illness-related stress.
In fact, verbal memory impairments might be related to the type of chemotherapy regimen. Our results suggested that women with BC who underwent adjuvant CMF chemotherapy showed significantly lower prefrontal cortex activation during memory encoding than women treated with ACT chemotherapy. As shown in Figure 2, women with BC who received ACT chemotherapy were more similar to controls in terms of prefrontal activation. Additionally, 3 of the 5 women treated with CMF actually showed deactivation of the prefrontal cortex (negative mean ROI value) during encoding. CMF rather than ACT was associated with greater brain activation impairment likely because of methotrexate. Previous studies implicate an association between cognitive deficits and methotrexate in women with BC (17), an animal model suggested negative effects of methotrexate on the frontal lobe functions in rats (23), and one study showed abnormal prefrontal cortex morphology in children with leukemia treated with methotrexate (41). However, our results should be considered preliminary given the small sample size, particularly of the CMF group. Additionally, CMF is an older chemotherapy regimen that is no longer as commonly utilized as other regimens (women in our sample treated with CMF underwent chemotherapy in the late 1990’s). Therefore, this finding may have clinical relevance only for women who underwent chemotherapy in the more remote past.
It is unclear why CMF chemotherapy was associated with brain activation during encoding but not recall. Between-group activation differences during recall were much more diffuse than during encoding, which may have reduced the power of the recall ROI analysis. As indicated above, methotrexate has been associated with prefrontal cortex abnormalities. Although both encoding and recall tasks were associated with significant between-group differences in prefrontal cortex, encoding differences were more in the left hemisphere while recall differences were more in the right. This is consistent with previous studies demonstrating a left-sided bias for encoding and a right-sided bias for retrieval (42). Children with leukemia who received methotrexate demonstrated more significant left prefrontal abnormalities (40). Thus, left prefrontal cortex regions may have increased vulnerability to methotrexate-related toxicity. This potential leftward vulnerability may stem from the numerous well-documented left-right asymmetries of the prefrontal cortex including lower white matter volume (43) and coherence (44) of the left frontal hemisphere. Additionally, the left frontal cortex has significantly lower N-acetylaspartate (NAA) levels than the right. NAA is believed to play a role in glial cell-specific signaling and myelination (45). Methotrexate is known to cause white matter abnormalities (46, 47). The left frontal cortex may have less white matter “reserves” to deal with neurotoxic injury than the right due to the asymmetries described above.
Tamoxifen did not predict brain activation in regions of between-group difference but there was not enough variance to truly explore this relationship given that only 3 women out of 14 did not receive tamoxifen. Future studies should include a second comparison group of women with BC treated only with surgery as many of these receive tamoxifen or other similar hormonal therapies, but not chemotherapy. The addition of this “no chemotherapy” group would also help address the weakness of the present study inherent in cross-sectional designs. Specifically, based on the present results, it is difficult to determine if the altered brain activation profiles in the BC group stem from chemotherapy effects, additional aspects of BC (e.g. immunosuppression, inflammatory responses), or a combination of these factors. In fact, some studies have suggested that a percentage of women with BC may have cognitive impairments - including verbal memory deficits - prior to beginning adjuvant chemotherapy (14).
In conclusion, our present findings provide preliminary but compelling evidence of neurofunctional deficits associated with verbal declarative memory in women with metastatic breast cancer, potentially specific to those treated with CMF chemotherapy. Our results have important implications not only for chemotherapy treatments in BC but also for potential cognitive interventions in women with BC who demonstrate verbal memory deficits. However, small sample sizes and a cross-sectional design limit the interpretation of findings. Larger studies utilizing neuroimaging techniques with appropriate comparison groups and/or longitudinal designs are necessary to further elucidate the neurobiologic status associated with cognitive deficits in women with BC.
TRANSLATIONAL RELEVANCE
These findings provide neuroanatomically specific evidence of neurofunctional deficits associated with memory storage and retrieval in women with metastatic breast cancer, potentially specific to those treated with CMF chemotherapy. These results suggest that certain types of chemotherapy may induce verbal memory impairment as demonstrated by altered functional brain activation profiles. They provide evidence regarding the oft-cited but poorly understand syndrome of ‘chemobrain’ among women with breast cancer, explaining their primary neurocognitive problem as difficulty in performing cognitive tasks rather than deficits in the outcome of their performance per se. They also indicate that one type of chemotherapy, CMF, was most strongly associated with these deficits, suggesting opportunities for adjusting chemotherapy to minimize neurocognitive impairment and/or targeting women who received this older regimen for cognitive interventions. These results have important implications not only for chemotherapy treatments in breast cancer, but also for the design of cognitive intervention programs for women with BC who demonstrate verbal memory deficits.
ACKNOWLEDGEMENTS
This study was supported by NIA/NCI Program Project grant AG18784 (D.S.), the Randolph H. Chase, M.D., Fund II (D.S.), The Jay and Rose Phillips Family Foundation (D.S.) and the NIH Director’s New Innovator Award 1DP2OD004445-01 (S.K.). The authors would like to thank Bita Nouriani for helping with recruitment coordination, Eric Neri for his assistance with cortisol and psychosocial data, and Nancy Adleman for her help with fMRI task programming and testing.
Footnotes
REFERENCES
- 1.Burstein HJ. Cognitive side-effects of adjuvant treatments. Breast. 2007;16 Suppl 2:S166–S168. doi: 10.1016/j.breast.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2007;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 3.Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19:623–627. doi: 10.1097/CCO.0b013e3282f0e224. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy J. Chemotherapy-related cognitive dysfunction in breast cancer. Semin Oncol Nurs. 2003;19:17–24. doi: 10.1053/j.soncn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2006 doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 7.Abraham J, Haut M, Moran M, Filburn S, Lemiuex S, Kuwabara H. Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer. 2008;8:88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Esposito M. Functional neuroimaging of cognition. Seminars in neurology. 2000;20:487–498. doi: 10.1055/s-2000-13182. [DOI] [PubMed] [Google Scholar]
- 10.Kotsoni E, Byrd D, Casey BJ. Special considerations for functional magnetic resonance imaging of pediatric populations. Journal of magnetic resonance imaging : JMRI. 2006;23:877–886. doi: 10.1002/jmri.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poldrack RA, ParÇ-Blagoev EJ, Grant PE. Pediatric functional magnetic resonance imaging: progress and challenges. Topics in magnetic resonance imaging : TMRI. 2002;13:61–70. doi: 10.1097/00002142-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wilke M, Holland SK, Myseros JS, Schmithorst VJ, Ball WS. Functional magnetic resonance imaging in pediatrics. Neuropediatrics. 2003;34:225–233. doi: 10.1055/s-2003-43260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strangman G, O'Neil-Pirozzi TM, Burke D, et al. Functional neuroimaging and cognitive rehabilitation for people with traumatic brain injury. Am J Phys Med Rehabil. 2005;84:62–75. doi: 10.1097/01.phm.0000150787.26860.12. [DOI] [PubMed] [Google Scholar]
- 14.Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. 'Chemobrain' in breast carcinoma?: a prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 15.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: Analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, van Dam FS. Electrophysiological correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2005;94:53–61. doi: 10.1007/s10549-005-7093-3. [DOI] [PubMed] [Google Scholar]
- 18.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 19.Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of Tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J Cancer Surviv. 2008;2:275–282. doi: 10.1007/s11764-008-0070-1. [DOI] [PubMed] [Google Scholar]
- 20.Schilder CM, Eggens PC, Seynaeve C, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich J, Han R, Yang Y, Mayer-Prîschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacology, biochemistry, and behavior. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Irwin M, Daniels M, Risch SC, Bloom E, Weiner H. Plasma cortisol and natural killer cell activity during bereavement. Biological psychiatry. 1988;24:173–178. doi: 10.1016/0006-3223(88)90272-7. [DOI] [PubMed] [Google Scholar]
- 25.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger DA, Schwartz GE. Distress and restraint as superordinate dimensions of self-reported adjustment: a typological perspective. J Pers. 1990;58:381–417. doi: 10.1111/j.1467-6494.1990.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher D, Nies G, Thompson LW. Reliability of the Beck Depression Inventory with older adults. J Consult Clin Psychol. 1982;50:152–153. doi: 10.1037//0022-006x.50.1.152. [DOI] [PubMed] [Google Scholar]
- 29.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 30.Greicius MD, Krasnow B, Boyett-Anderson JM, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- 31.Mazaika P, Whitfield-Gabrielli S, Reiss AL. Aritfact repair for fMRI data from high motion clinical subjects. Human Brain Mapping. 2007:2007. [Google Scholar]
- 32.Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. J Cereb Blood Flow Metab. 1995;15:361–370. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- 33.Holmes AP, Friston KJ. Generalisability, random effects, and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- 34.Menon V, Boyett-Anderson JM, Schatzberg AF, Reiss AL. Relating semantic and episodic memory systems. Brain Res Cogn Brain Res. 2002;13:261–265. doi: 10.1016/s0926-6410(01)00120-3. [DOI] [PubMed] [Google Scholar]
- 35.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 36.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 37.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 38.Weis J, Poppelreuter M, Bartsch HH. Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: 'subjective' complaints and 'objective' neuropsychological test results. Psychooncology. 2008 doi: 10.1002/pon.1472. [DOI] [PubMed] [Google Scholar]
- 39.O'Hara R, Yesavage JA, Kraemer HC, Mauricio M, Friedman LF, Murphy GM., Jr The APOE epsilon4 allele is associated with decline on delayed recall performance in community-dwelling older adults. J Am Geriatr Soc. 1998;46:1493–1498. doi: 10.1111/j.1532-5415.1998.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 40.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-oncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 41.Lesnik PG, Ciesielski KT, Hart BL, Benzel EC, Sanders JA. Evidence for cerebellar-frontal subsystem changes in children treated with intrathecal chemotherapy for leukemia: enhanced data analysis using an effect size model. Arch Neurol. 1998;55:1561–1568. doi: 10.1001/archneur.55.12.1561. [DOI] [PubMed] [Google Scholar]
- 42.Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci U S A. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zilles K, Dabringhaus A, Geyer S, et al. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 44.Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 45.Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- 46.Fisher MJ, Khademian ZP, Simon EM, Zimmerman RA, Bilaniuk LT. Diffusion-weighted MR imaging of early methotrexate-related neurotoxicity in children. AJNR Am J Neuroradiol. 2005;26:1686–1689. [PMC free article] [PubMed] [Google Scholar]
- 47.Linnebank M, Moskau S, Jurgens A, et al. Association of genetic variants of methionine metabolism with methotrexate-induced CNS white matter changes in patients with primary CNS lymphoma. Neuro Oncol. 2009;11:2–8. doi: 10.1215/15228517-2008-082. [DOI] [PMC free article] [PubMed] [Google Scholar]