Abstract
Visual masking deficit in schizophrenia has been suggested to be a potential vulnerability marker for schizophrenia. An important characteristic of a vulnerability marker is stability over time, but relatively little is known about the longitudinal course of masking performance of schizophrenia patients. In this study, we examined the stability of visual masking performance in recent-onset schizophrenia patients over an 18-month period. We administered both forward and backward masking trials with multiple stimulus onset asynchronies for four masking conditions at three time points (baseline, 6-month, and 18-month). Recent-onset schizophrenia patients showed stable masking performance for both forward and backward conditions over a period of 18 months. Furthermore, the stable performance was observed across all four masking conditions. The findings of this study provide further support for the view that visual masking deficits reflect a possible vulnerability marker for schizophrenia.
Keywords: visual masking, stability, vulnerability, recent-onset schizophrenia, longitudinal study
INTRODUCTION
Perceptual processing deficits are commonly observed in schizophrenia, and one of the most thoroughly investigated perceptual tasks in schizophrenia is visual masking. In visual masking, the ability to identify a visual target is disrupted by the presence of a mask that occurs briefly before or after the target (Breitmeyer, 1984; Breitmeyer and Ogmen, 2000). Forward masking occurs when a mask precedes a target; backward masking occurs when a mask follows a target. It has long been noted that schizophrenic patients have more difficulty than comparison subjects in identifying the target in the presence of a mask (Saccuzzo and Braff, 1981; Green and Walker, 1986; Rund, 1993; Cadenhead et al., 1998).
Visual masking performance is frequently viewed as the interaction between two key visual channels that form the basis for complex visual processing (Van Essen et al., 1992). The two pathways are known by the functionally based terms “sustained channel” and “transient channel”, or by the anatomically based terms “parvocellular pathway” and “magnocellular pathway”. According to this model, the sustained or parvocellular activity elicited by a target conveys detailed information that is needed to identify targets, while the transient or magnocellular activity provides more rapid information that allows location of a stimulus but not identification. Masking results from disruption of this target information by the magnocellular or parvocellular pathways of the mask (Breitmeyer and Ganz, 1976; Breitmeyer, 1984).
Based on this model, visual masking procedures have also been used to explore dysfunctional neural circuits in schizophrenia. Findings from several laboratories have implicated the magnocellular pathway in the visual masking deficit in schizophrenia (Schuck and Lee, 1989; Green et al., 1994; Cadenhead et al., 1998; Schechter et al., 2003). Aside from the magnocellular system, abnormalities in the parvocellular system have also been proposed for schizophrenia. Purushothaman and colleagues (2000) proposed that gamma range activity is a feature of the parvocellular pathways. Along these lines, visual masking tasks have been used to detect gamma range abnormalities in schizophrenia (Green et al., 2003b; Wynn et al., 2005).
Moving out from neural circuits to everyday functioning, visual masking deficits have been linked to performance on a measure of social perception (i.e., ability to perceive a social context from brief vignettes, Sergi and Green, 2002) and have served as a key component of a model of community functioning (Sergi et al., 2006). Hence, improved understanding of visual masking deficits in schizophrenia may provide a useful lynchpin in mapping pathways from neural circuits to community functioning.
Emerging evidence suggests that performance on visual masking tasks may be a trait marker that reflects vulnerability to schizophrenia rather than a reflection of a symptomatic state. For example, masking deficits have been reported in schizophrenia patients who are in clinical remission (Miller et al., 1979; Green et al., 1999). In addition, first-degree relatives of schizophrenia patients often show similar, but milder, masking deficits than patients (Green et al., 1997; Keri et al., 2001; Green et al., 2006), though specific task parameters may affect the magnitude of the group differences (e.g., Bedwell et al., 2003). Similarly, masking deficits have been observed in individuals who are considered to be psychosis-prone (Merritt and Balogh, 1989; Cadenhead et al., 1996).
Beyond these demonstrations that visual masking deficits occur in individuals who are at heightened risk for schizophrenic episodes but who are not currently symptomatic, a reasonable degree of stability over time would be expected if visual masking deficits are vulnerability markers for schizophrenia (Zubin and Spring, 1977; Nuechterlein and Dawson, 1984; Nuechterlein et al., 1994). Surprisingly, however, little is known about the stability of masking performance deficits in schizophrenia.
Only one study to our knowledge examined visual masking in schizophrenia over an extended follow up period. Rund et al. (1993) reported no significant effect of time on a backward masking performance in a sample of 22 schizophrenia patients tested over a 2-year period. Although this study suggests that visual masking performance in schizophrenia is relatively stable over a 2-year period, the conclusions are limited by the use of a very basic masking procedure (i.e. only two stimulus onset asynchronies, limited temporal resolution, no forward masking, and only a single high-energy condition). The study also included patients with a wide range of ages and illness chronicity, so it is difficult to determine whether the stability suggested in this initial study applies to the initial phase of schizophrenia in which clinical fluctuations are often more marked over time.
The goal of the current study was to examine in a thorough manner the stability of visual masking performance in schizophrenia. To do so, we prospectively assessed a sample of recent-onset schizophrenia patients at baseline, 6 months, and 18 months. The visual masking procedures included multiple SOAs for forward and backward masking for four different masking conditions that differed in the extent to which they relied on the magnocellular and parvocellular pathways.
METHODS
Participants
Participants included 42 patients (14 females) who met Research Diagnostic Criteria (RDC; Spitzer et al., 1978) for either schizophrenia or schizoaffective disorder. All were participants in the third phase of the Developmental Processes in Schizophrenic Disorders Project (PI: K. H. Nuechterlein), an 18-month follow-through longitudinal study of schizophrenia patients who had experienced a first episode of psychosis within 2 years of project entry (Nuechterlein et al., 1992; Nuechterlein et al., in press). Participants were recruited from a variety of local Los Angeles area psychiatric hospitals and outpatient clinics. Selection criteria for this study included: 1) a recent onset of psychotic illness, with the beginning of the first major psychotic episode occurring within the last 2 years; 2) a diagnosis of schizophrenia or schizoaffective disorder, mainly schizophrenic subtype by RDC (Spitzer et al., 1978); 3) between 18 and 45 years of age; 4) no evidence of a known neurological disorder; 5) no evidence of significant substance abuse or alcoholism in the 6 months prior to study entry 6) no history of mental retardation; 7) sufficient fluency in English to understand study instructions; 8) living within commuting distance of the UCLA Aftercare Program; 9) expressed interest in returning to work or school; 10) treatment with risperidone (which was part of study protocol) should not be contraindicated. Participants were receiving psychosocial and psychopharmacological outpatient psychiatric treatment at the UCLA Aftercare Program at the time of testing. The Institutional Review Board of UCLA approved the research protocol. All participants were provided with oral and written information about the research procedures involved in the study and gave written informed consent.
The baseline assessment for this study occurred during a period of outpatient clinical stabilization. Stabilization was defined in terms of being on a maintenance dosage of risperidone rather than in specific symptom severity terms, and typically occurred two to three months after entry into the outpatient clinic when the acute symptoms were resolving. A small number of these subjects (n = 11) also received a masking assessment at clinic entry when they were acutely symptomatic, as part of a sub-protocol. Because so few subjects received assessment in this initial acute state, we did not use these data in the analyses.
Design and Procedure
We administered the visual masking task on three occasions: baseline following clinical stabilization, 6 months, and 18 months. The masking tasks included the four masking conditions described below: target location with a high-energy mask, target identification with a high-energy mask, target identification with a low-energy mask, and target identification with paracontrast/metacontrast mask.
Stimuli used in the visual masking task are presented in Figure 1. The target was a square with a gap on one of three sides (up, down, or left) that could appear in any one of four locations (upper left, upper right, lower left, lower right). Each target location was 1.03° (degrees of visual angle) from fixation and each target subtended 0.27°. The mask for the first three conditions was a composite square made up of four smaller squares and appeared at each of the four possible target locations. The paracontrast/metacontrast mask was a square that surrounded, but did not overlap, all possible areas where the target could appear.
Figure 1.
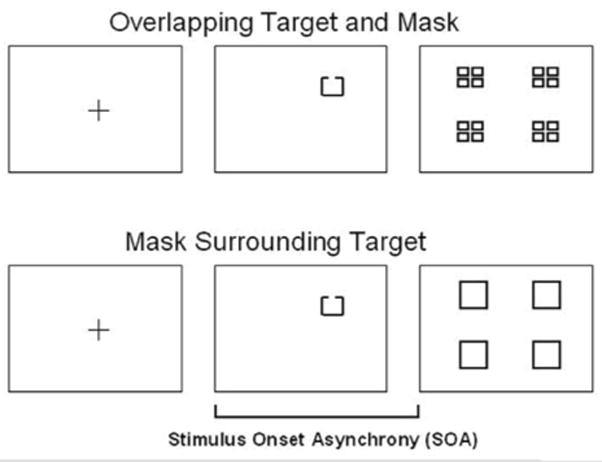
Masking stimuli. The target for all conditions was a target with a gap on one of three sides (up, down, or left) that could appear in any one of four locations (upper left, upper right, lower left, lower right). The mask for three masking conditions (location with a high-energy mask, identification with a high-energy mask, identification with a low-energy mask) was a composite square made up of four smaller squares that covered all possible target locations. The mask for paracontrast/metacontrast condition was a square that surrounded, but did not overlap, all possible target locations.
The masking methods are described in detail elsewhere (Green et al., 2003a; Rassovsky et al., 2004). Before completing the visual masking task, participants were equated at 84% accuracy for unmasked performance using a psychophysical staircase method (Wetherill and Levitt, 1965). A target was presented for 13.3 ms (two screen sweeps at 150 Hz) for the staircase procedure and all subsequent masking procedures. During the thresholding procedure, the contrast of the target (i.e., the gray value scale) was systematically increased or decreased based on participants’ performance to achieve 84% accuracy. This contrast level was used for all subsequent masking procedures. Intervals between target and mask were measured by stimulus onset asynchronies (SOAs), defined as the time interval between the onset of the target and the mask.
At the beginning of each trial, a fixation point was presented for 300 ms, followed by 100 ms of blank screen, before the target onset. For backward masking, the target was followed by the mask and, for forward masking, the target was preceded by the mask. For each masking condition, twelve trials were presented for each SOA, so that three targets and four locations could be counterbalanced. There were also 12 unmasked trials in each masking condition.
The four masking conditions (see below for detailed description) rely to differing degrees on magnocellular and parvocellular pathways (Breitmeyer, 1984; Breitmeyer and Ogmen, 2000). The location condition places more reliance on the magnocellular channels. The first two conditions generate a “monotonic” masking function in which performance increases with increasing SOA for backward masking. The last two conditions typically generate non-monotonic masking functions in which the backward masking portion is U-shaped. U-shaped masking functions can occur when the mask is relatively weak compared to the target (e.g., the low-energy condition), or when the mask does not spatially overlap the area of the target (e.g., para- metacontrast). A U-shaped masking function suggests that primary masking mechanism involves interruption (i.e., the disruption of the parvocellular pathways of the target by the magnocellular pathway of the mask) as opposed to integration (i.e., fusing of the parvocellular pathway activity from both target and mask). The four masking conditions included:
Target location with a high-energy mask: In this condition, participants did not need to identify the target; instead they indicated where the target appeared. The energy of the mask was twice that of the target (four screen sweeps for the mask; two for the target). The term ‘energy’ in visual masking refers to the product of the duration and intensity of a stimulus.
Target identification with a high-energy mask: In this condition, participants were asked to identify the target. The energy of the mask was twice the energy of the target (four screen sweeps for the mask; two for the target).
Target identification with a low-energy mask: In this condition, the energy of the mask was half the energy of the target (one screen sweep for the mask; two for the target).
Target identification with Paracontrast/Metacontrast mask: In this condition, the energy of the task was equal to that of the target (two screen sweeps for the mask: two for the target).
The staircase procedure took approximately 10 minutes to administer and the masking conditions took approximately 15 minutes each. Typically, all conditions were administered during the same session, but participants could request breaks.
Statistical Analyses
We examined the stability of visual masking performance in schizophrenia patients over an 18-month period (i.e., the absence of significant change over time) using two statistical analyses. First, a repeated measures ANOVA was performed to test for changes in the mean level of performance over time. Second, Spearman rank correlations were used to examine the consistency of rank order of performance over time. The key dependent measures were the average accuracy over SOAs for forward and backward masking trials for each masking condition. For the repeated measures ANOVA, we included 3 time points (baseline, 6, and 18 months), 4 masking conditions, and forward/backward masking as within-subject variables and analyzed data from 28 subjects with complete data at all three time points. Patients tended to miss testing more at 18 months than the other time points, leading to concerns about non-ignorability and bias if subjects with missing data were included. The Spearman rank correlation was performed across time points (baseline vs. 6-month, baseline vs. 18-month) for forward/backward of each masking condition. For the correlations, we used subjects if they had completed a baseline masking assessment and at least one other masking assessment (at 6 and/or 18 months) (n = 42).
RESULTS
The 42 participants had a mean age of 24.0 years (SD=3.5), a mean education of 13.4 years (SD=1.8), and a mean parental education of 14.6 years (SD=4.1). Clinical symptoms were rated using the expanded 24-item version of the Brief Psychiatric Rating Scale at baseline (BPRS; Ventura et al., 1993a; Ventura et al., 1993b), with a range of 1–7 per item. The mean total BPRS score was 35.7 (SD=9.4). The mean score for positive symptoms (Thought Disturbance factor; mean of unusual thought content, hallucination, and conceptual disorganization) was 2.4 (SD=1.2). The mean score for negative symptoms (Withdrawal-Retardation factor; mean of blunted affect, emotional withdrawal, and motor retardation) was 2.7 (SD=1.2).
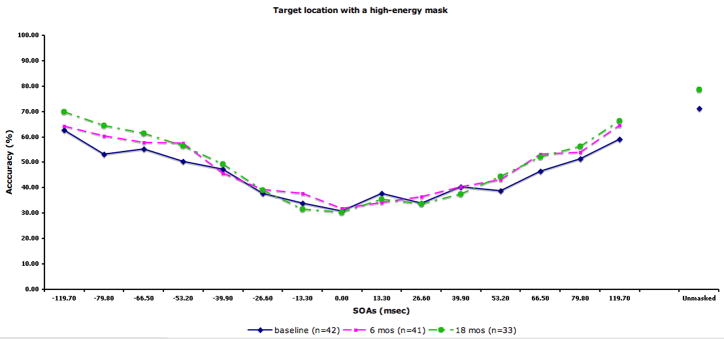
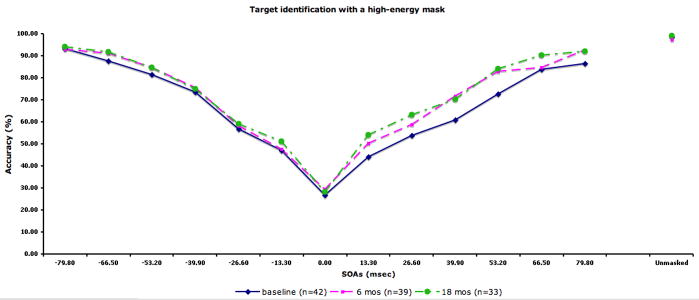
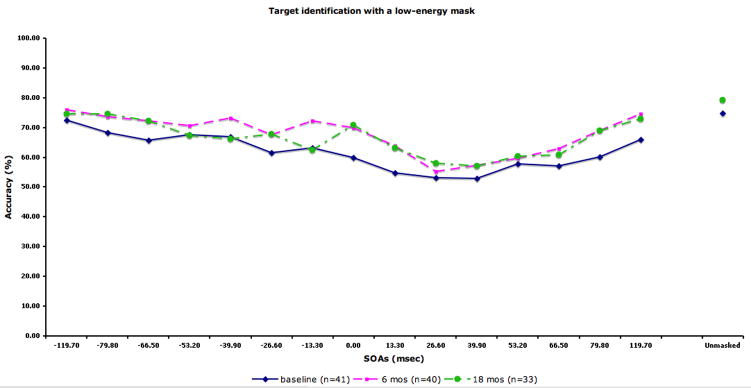
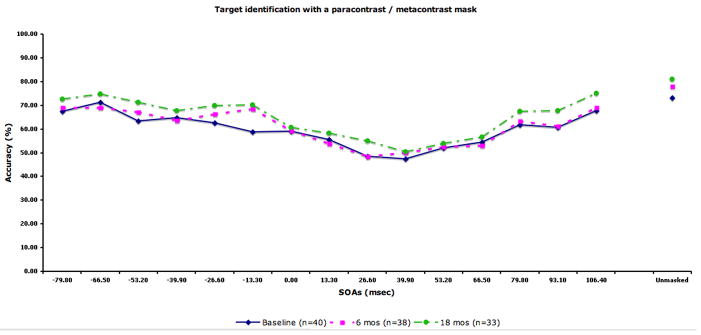
The performance data by SOA for each condition are displayed in Fig 2. The figure also includes the sample size at each occasion. We compared the 28 patients with complete data (i.e. tested at all three time points) to 14 patients who did not participate at all three time points (typically they had baseline plus 6 months). The two groups did not differ in terms of age, years of education, parental education, or masking performance at baseline.
Figure 2.
Performance data by SOA for each masking condition. The solid line indicates performance at baseline. The dotted lines with squares and circles indicate performance at 6-month and 18-month follow-up, respectively. A) Location with a high-energy mask. Chance performance for this condition is 25% accuracy. B) Identification with a high-energy mask. C) Identification with a low-energy mask. D) Identification with paracontrast/metacontrast mask. For the three identification conditions, chance performance is 33% accuracy.
In the repeated measures ANOVA, we found a significant effect of masking condition (F3,81=68.58, p<.001), a significant effect of forward/backward (F1,27=37.45, p<.001), a significant masking condition by forward/backward interaction (F3,81=5.05, p<.01), and a significant masking condition by time by forward/backward interaction (F6,162=3.22, p<.01). The main effect for time was not significant, indicating that masking performance did not change over time. For the masking conditions, each condition was significantly different from each other in the following order of increasing performance: the high-energy mask condition, low-energy mask condition, metacontrast/paracontrast condition, and target location. Patients showed overall better performance for forward masking compared with backward. The condition by forward/backward interaction was due to a larger performance difference between forward and backward masking for the three target identification conditions compared with the location condition. The three-way interaction was not predicted, and not easily interpretable.
We further investigated the stability of the masking performance in schizophrenia patients by computing the Spearman rank correlation (see Fig. 3) between testing points (baseline vs. 6-month and baseline vs. 18-month). For all masking conditions, we found positive correlations between baseline and 6 month testing (ranging from .231 – .685) and between baseline and 18-month testing (ranging from .276 – .702). Almost all of these correlations were moderate to strong and were statistically significant, except for the .231 and .276 correlations for backward masking in the identification condition with a high-energy mask. Furthermore, these low correlations for backward masking in the identification condition with a high-energy mask were not significantly different from correlations in other conditions (Raghunathan et al., 1996).
Figure 3.
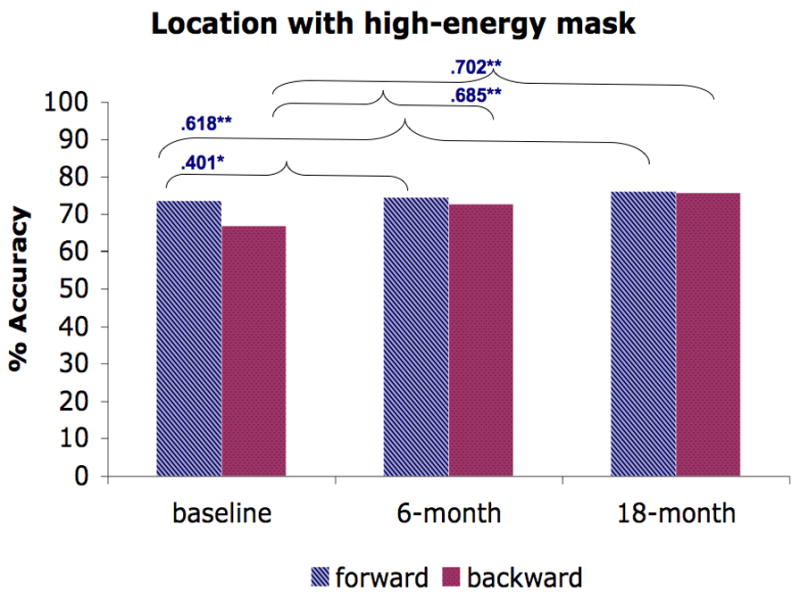
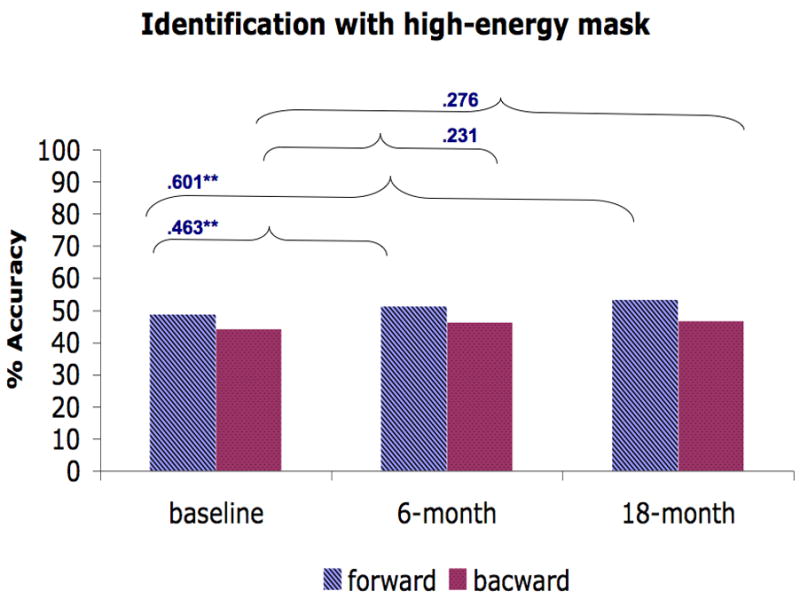
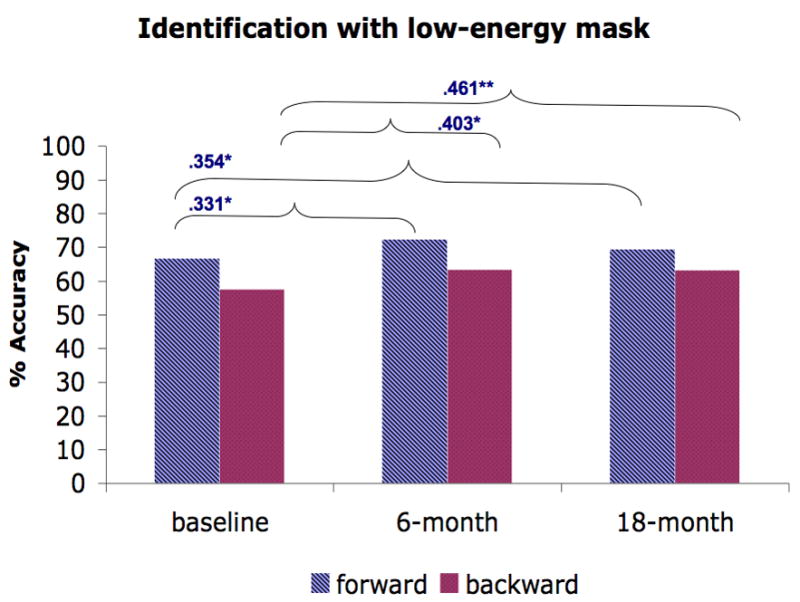
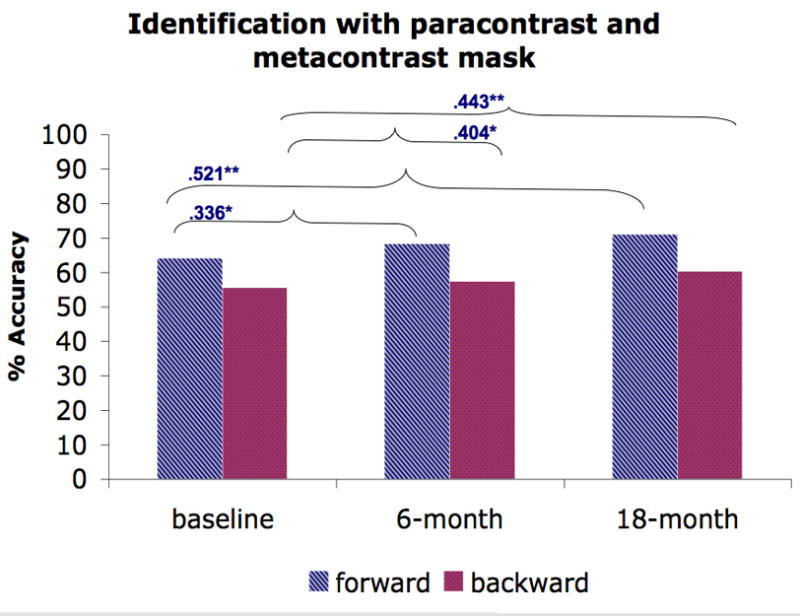
The average performance of forward and backward masking trials for each masking condition and the Spearman rank correlation across time points (baseline vs. 6-month, baseline vs. 18-month). A) Location with a high-energy mask. B) Identification with a high-energy mask. C) Identification with a low-energy mask. D) Identification with paracontrast/metacontrast mask. * indicates a significant p-value at .05 and ** indicates a significant p-value at .01.
DISCUSSION
In this study, we investigated the stability of forward and backward visual masking performance across four different masking conditions in recent-onset schizophrenia patients over a period of 18 months. The patients showed relatively stable performance (i.e. no effect of time) and this pattern was consistent across forward and backward masking, and across the four masking conditions. In addition, the rank order of performance was relatively consistent from baseline to 6 months, and from baseline to 18 months.
The current study is consistent with a previous study that examined visual masking stability in a smaller sample of patients with mixed chronicity (Rund et al., 1993). The current study expands upon previous findings in the following ways: 1) it included only recent-onset schizophrenia patients, 2) it showed stable performance on forward, as well as backward, masking, 3) the stability was consistent across masking conditions that differ in the degree to which they rely on the magnocellular versus parvocelluar pathways. We previously demonstrated masking deficits in recent onset, unmedicated patients (Green et al., 1999). The current results suggest that impaired visual masking performance at this early stage remains quite stable over a period of 18 months.
For the Spearman correlations that assess stability between occasions, it is ironic that the lowest correlations were observed for the identification condition with a high-energy mask, as this condition is typical of the visual masking paradigm that has been most commonly used in the literature on schizophrenia. The stability of performance in this condition is not significantly lower than in the other conditions, so this may represent a chance fluctuation. On the other hand, the tendency toward higher stability in other conditions suggests the value of continued study of multiple masking conditions when an index of a stable vulnerability factor is the goal.
The stability over time demonstrated in this study is conceptually consistent with a recent study of attentional modulation of visual masking in schizophrenia (Rassovsky et al., 2005). Their study found that “top-down” attentional manipulation by monetary reward had only modest effect on visual masking performance in schizophrenia patients. The conclusion from both of these studies is that visual masking performance is tapping into perceptual processes that do not change easily, either on their own over time, or momentarily with incentives.
The study had some limitations. The patients were all taking antipsychotic medications. However, other studies have shown that visual masking impairment is not simply a result of medications (Miller et al., 1979; Braff, 1981; Green et al., 1999). Although our sample was larger than the one in the previous study that examined masking stability, our sample sizes were still modest due to focusing on only recent-onset schizophrenia patients. A larger sample may have been able to detect smaller changes over time. Finally, because this study did not include normal controls, we do not know if improvement over time would be expected in a non-clinical sample.
In summary, this study demonstrated that, early in the course stage of illness, schizophrenia patients show stable visual masking performance over an 18-month period across multiple masking conditions. This pattern of stability over time provides added support that visual masking is a likely vulnerability marker for schizophrenia.
Acknowledgments
We thank Jonathan K. Wynn and the Aftercare Research Program staff for their valuable comments and help.
Role of the Funding Source
This work is supported by grants MH43292 to Dr. Green and MH66286 and MH37705 to Dr. Nuechterlein.
Footnotes
Contributions
Junghee Lee was responsible for drafting this paper and obtaining input from the co-authors. Michael F. Green and Keith H. Nuechterlein designed the study and supervised administration of the study protocol. Catherine A. Sugar was primarily responsible for statistical analyses. Denise Gretchen-Doorlyand Kimberly Kelly supervised the data collection. Kenneth Subotnik and Joseph Ventura, along with all of the authors, contributed to, and have approved, the final manuscript.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- Bedwell JS, Brown JM, Miller S. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophrenia Research. 2003;63:273–284. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- Braff DL. Impaired speed of information processing in nonmedicated schizotypal patients. Schizophrenia Bulletin. 1981;7:499–508. doi: 10.1093/schbul/7.3.499. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG. Visual masking: An integrative approach. New York: Oxford University Press; 1984. [Google Scholar]
- Breitmeyer BG, Ganz L. Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processing. Psychological Review. 1976;83:1–36. [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: A comparison, review, and update. Perception and Psychophysics. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Perry W, Braff DL. The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biological Psychiatry. 1996;40:853–858. doi: 10.1016/0006-3223(95)00547-1. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the VBM deficits of schizophrenia patients. Biological Psychiatry. 1998;43:132–138. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Green MF, Walker E. Symptom correlates of vulnerability to backward masking in schizophrenia. American Journal of Psychiatry. 1986;143:181–186. doi: 10.1176/ajp.143.2.181. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54:465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59:446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Tsuang J, Mintz J. Forward and backward visual masking in schizophrenia: influence of age. Psychol Med. 2003a;33:887–895. doi: 10.1017/s003329170200716x. [DOI] [PubMed] [Google Scholar]
- Green MF, Mintz J, Salveson D, Nuechterlein KH, Breitmeyer B, Light GA, Braff DL. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003b;53:1113–1119. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: A neurocognitive approach. Psychological Medicine. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- Merritt RD, Balogh DW. Backward masking spatial frequency effects among hypothetically schizotypal individuals. Schizophr Bull. 1989;15:573–583. doi: 10.1093/schbul/15.4.573. [DOI] [PubMed] [Google Scholar]
- Miller S, Saccuzzo D, Braff D. Information processing deficits in remitted schizophrenics. Journal of Abnormal Psychology. 1979;88:446–449. [PubMed] [Google Scholar]
- Nuechterlein K, Dawson M, Green M. Information-processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatrica Scandinavica. 1994;90:71–79. doi: 10.1111/j.1600-0447.1994.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophrenia Bulletin. 1984;10:300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Turner LR, Ventura J, Becker DR, Drake RE. Individual placement and support for individuals with recent-onset schizophrenia: Integrating supported education and supported employment. Psychiatric Rehabilitation Journal. doi: 10.2975/31.4.2008.340.349. (in press) [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental Processes in Schizophrenic Disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Ogmen H, Bedell HE. Gamma-range oscillations in backward masking functions and their putative neural correlates. Psychological Review. 2000;107:556–577. doi: 10.1037/0033-295x.107.3.556. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Rosenthal R, Rubin DB. Comparing corrleated but nonoverlapping correlations. Psychological Methods. 1996;1:178–183. [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Paracontrast and metacontrast in schizophrenia: clarifying the mechanism for visual masking deficits. Schizophr Res. 2004;71:485–492. doi: 10.1016/j.schres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. Am J Psychiatry. 2005;162:1533–1535. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- Rund BR. Backward-masking performance in chronic and nonchronic schizophrenics, affectively disturbed patients, and normal control subjects. J Abnorm Psychol. 1993;102:74–81. doi: 10.1037//0021-843x.102.1.74. [DOI] [PubMed] [Google Scholar]
- Rund BR, Landro NI, Orbeck AL. Stability in backward masking performance in schizophrenics, affectively disturbed patients, and normal subjects. J Nerv Ment Dis. 1993;181:233–237. doi: 10.1097/00005053-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia: New findings using schizophrenic subgroups and manic control subjects. Archives of General Psychiatry. 1981;38:175–179. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo GVZ, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schuck JR, Lee RG. Backward masking, information processing, and schizophrenia. Schizophrenia Bulletin. 1989;15:491–500. doi: 10.1093/schbul/15.3.491. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophrenia Research. 2002;59:233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: An integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters. International Journal of Methods in Psychiatric Research. 1993a;3:221–224. [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: Scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993b;3:227–243. [Google Scholar]
- Wetherill GB, Levitt H. Sequential Estimation of Points on a Psychometric Function. Br J Math Stat Psychol. 1965;18:1–10. doi: 10.1111/j.2044-8317.1965.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability - A new view of schizophrenia. Journal of Abnormal Psychology. 1977;86:103–126. doi: 10.1037//0021-843x.86.2.103. [DOI] [PubMed] [Google Scholar]