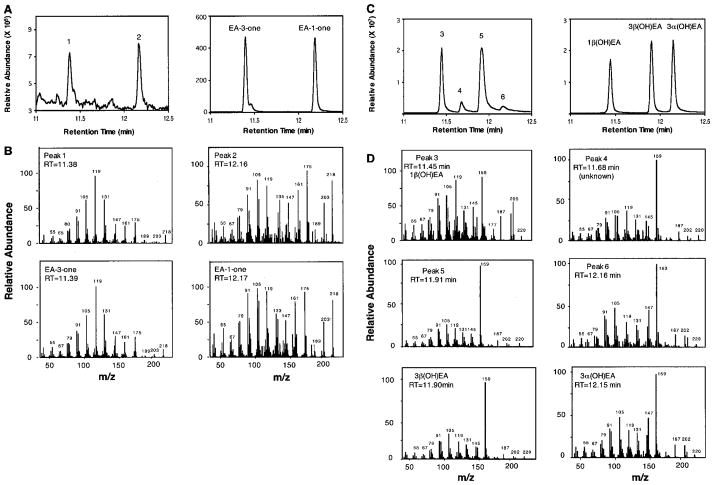
Fig. 6. Reaction product analysis of EAH-S368V incubated with EA.
EAH-S368V was incubated with 40 μM EA, and the total reaction products were fractionated by silica gel chromatography. Total ion chromatograms of the reaction products eluted from the silica gel with 5% ethyl acetate in hexane (A, left panel) are compared with those of authentic standards for EA-3-one and EA-1-one (A, right panel). Mass spectra for peaks 1 and 2 are compared directly with those for the respective ketones (B). Total ion chromatograms of reaction products sequentially eluted from the silica gel with 20% ethyl acetate in hexane (C, left panel) are compared with those of authentic standards for 1β(OH)EA, 3α(OH)EA, and 3β(OH)EA (C, right panel). The mass spectrum for peak 3 matches that for 1β(OH)EA (D), shown previously in Fig. 3. The mass spectra for peaks 5 and 6 are compared directly with those for 3β(OH)EA and 3α(OH)EA (D). However, peak 5 resolves into two nearly equal sized peaks by chiral GC analysis (Supplemental Fig. 2), with peak 5-1 corresponding to 3β(OH)EA and peak 5-2 to 2β(OH)EA (as determined by NMR analysis). RT, retention time.