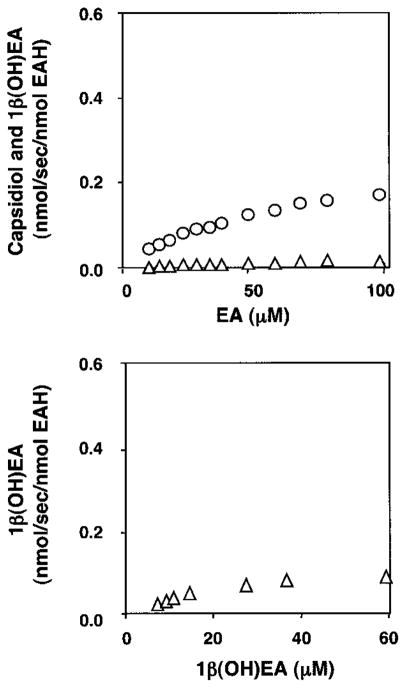
Fig. 7. Substrate-dependent activities of the EAH-I486A mutant.
The EAH-I486A mutation was created as described under “Experimental Procedures”; the mutant gene was expressed in yeast; and yeast microsomes were prepared as the source of the EAH-I486A enzyme. CO difference spectroscopy was used to qualify and normalize the amount of properly folded P450 enzyme used in the activity assays. Assays were incubated at the indicated concentrations of EA for 5 min (upper panel) or 1β(OH)EA for 1 min (lower panel) before profiling the reaction products by GC/MS. The only NADPH-dependent reaction products observed were capsidiol (△) and 1β(OH)EA (○). Capsidiol levels were near the detection limits for reactions incubated with EA.