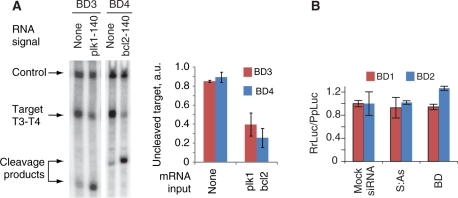
Figure 4.
The operation of biosensor devices in the Drosophila embryo lysate. (A) Autonomous operation of individual biosensors in lysate; 0.5 µl of 2-µM Pr3:As3 or 1-µM Pr4:As4 was added to a 7-µl mixture containing 5-µl lysate and ∼5-nM target RNAs, followed by immediate addition of 0.5 µl of 10-µM plk1-140nt or bcl2-140nt and 0.5 µl of 1-µM S3 or 0.5-µM S4, respectively. Equal aliquots of the corresponding S strand were added five additional times at 5-min intervals to compensate for the loss of S strand due to degradation by nucleases in the lysate. The mixture was additionally incubated at 25°C for 2 h. Cleavage of the target indicates the generation of the siRNA output, as expected in the presence of signal. Left panel, denaturing gel electrophoresis of the reaction mixture. Right panel, the quantified amount of the uncleaved target as measured in three replicates. (B) The assessment of the biosensor-induced perturbation of the mRNA signal. Full-length mRNA signals (100 nM RrLuc-Sig1 or RrLuc-Sig2) were incubated with 25 nM mock siRNA, 25 nM S:As siRNA outputs of their cognate biosensors or 25 nM biosensors themselves and then incubated in the Drosophila embryo lysate at 25°C for 2 h. The chart shows the relative translation of RrLuc-Sig1 and RrLuc-Sig2 as measured by the dual-luciferase assay (mean ± SD).