Figure 4.
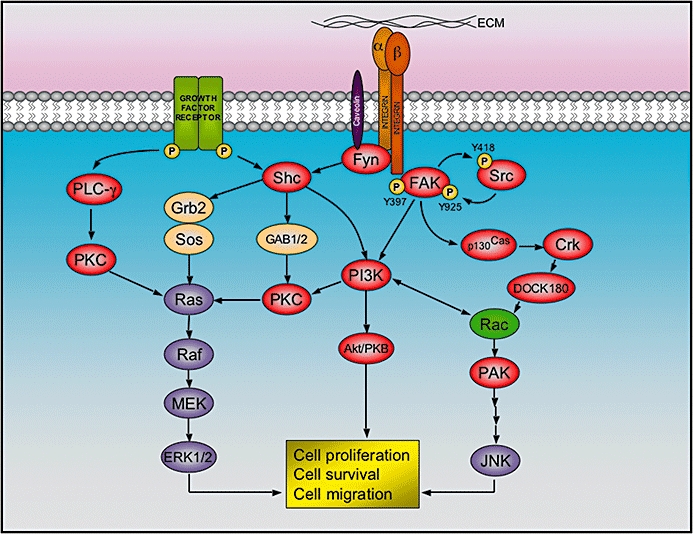
Integrin signalling at focal adhesions. After binding to the extracellular matrix (ECM), the cytoplasmic tail of integrin beta 1 recruits focal adhesion kinase (FAK), which is activated by auto-phosphorylation at the tyrosine 397, and thereby allow the binding and activation of Src by phosphorylation of tyrosine 418. The activated FAK/Src complex phosphorylates other kinases (i.e. p130Cas and PI3K), which in turn elicit a cascade of events that lead to cell proliferation, survival and migration. Alternatively, alpha subunits of certain integrins are able to recruit Fyn (a Src family member) via caveolin-1. This allows the activation of Shc, which combines with adaptor proteins Grb2 and SOS in order to activate the extracellular signal-regulated kinase (ERK)/MAP kinase pathway. As represented, the integrin pathway has reciprocal action with other signalling cascades triggered by several growth factor receptors.
