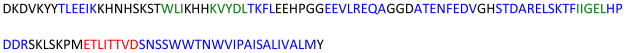Figure 1. The amino acid sequence of a full-length wild-type rabbit cytochrome b5.
High-resolution structures of the soluble domain of the protein have been reported from solution NMR and X-ray crystallography studies, whereas the structure of the full-length protein is unknown as it has not been amenable for studies using high-resolution methods. Secondary structures such as α-helix (blue) and βsheet (green) are indicated based on previous studies. The 8 amino acids that were deleted from the linker region of the wild-type cytochrome b5 to obtain a mutant are shown in red.