Table 3.
Enantioselective reduction of alkenyl and alkyl ketones.a
| entry | ketone | alcohol | catalyst | yieldb(%) | %eec |
|---|---|---|---|---|---|
| 1 |
27 |
28 |
D | 78 | 90 |
| 2 |
29 |
30 |
D | 88 | 86 |
| 3 |
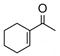 31 |
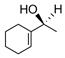 32 |
D | 82 | 97 |
| 4 |
33 |
34 |
D | 81 | 47 |
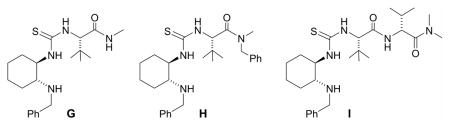
| 5 | 33 | 34 | G | 84 | 63 |
| 6 | 33 | 34 | H | 92 | 79 |
| 7 | 33 | 34 | I | 90 | 67 |
| 8 |
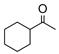 35 |
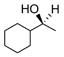 36 |
D | 60 | 89 |
| 9 | 35 | 36 | Hd | 68 | 91 |
Reaction conditions: catalyst (10 mol %), catecholborane (1.6 equiv), 4 Å molecular sieves, toluene, −46 °C, 24 h.
Isolated yield.
Measured by chiral HPLC.
36 h.