Figure 1.
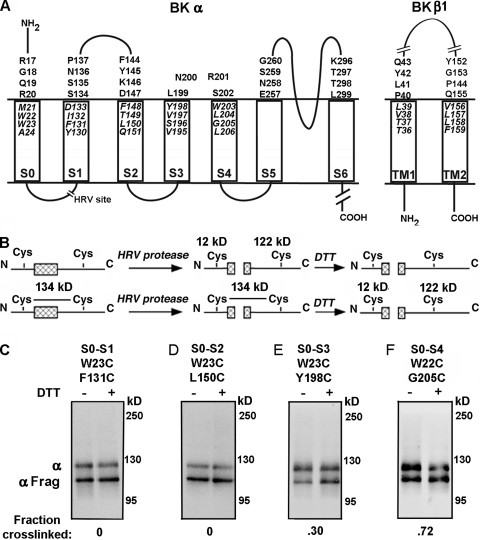
Intra–α subunit disulfide cross-linking from S0 to S1 through S4. (A) Membrane topology of BK α and β1 showing residues mutated to Cys in the predicted first helical turns within the membrane and in the extracellular flanks of the TM helices. The HRV-3C protease cleavage site in the S0–S1 loop is shown as a break. (B) Strategy to determine the extent of disulfide bond formation between S0 and S1 through S4 using HRV-3C protease and DTT. (C–F) The cell surface–expressed double-Cys mutant of α indicated at the top of each immunoblot was treated with HRV-3C protease alone (first lane) or protease followed by DTT (second lane). The immunoblots were developed with an antibody against a C-terminal epitope of α. The extent of cross-linking is indicated under each blot.