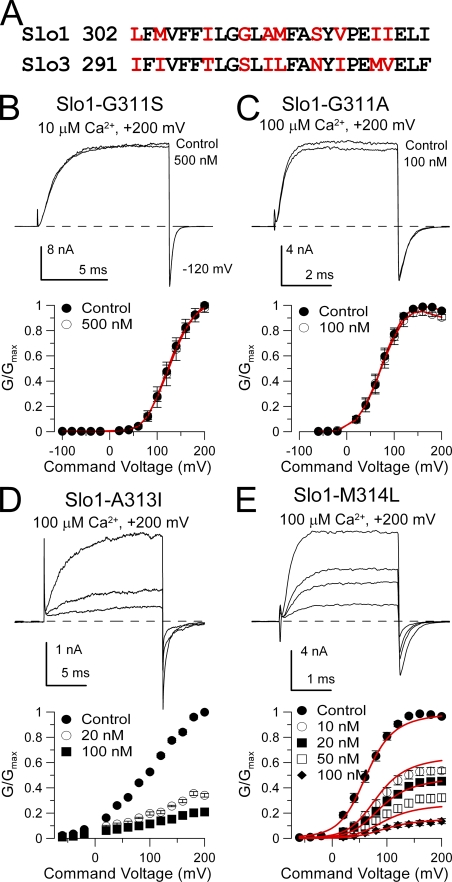
Figure 6.
Mutation of glycine 311 in Slo1 S6 abolishes high affinity block by paxilline. (A) A consensus alignment of Slo1 and Slo3 S6 segments is shown. Non-conserved residues are in red. (B) Paxilline is without effect on Slo1-G311S. Currents were activated with 10 µM Ca2+ at +200 mV, and 500 nM paxilline produces no effect. On the bottom, G-V curves for Slo1-G311S with and without 500 nM paxilline (n = 3 patches) are plotted. (C) Paxilline is without effects on Slo1-G311A. Currents were activated with 10 µM Ca2+ at +200 mV, and 500 nM paxilline had only a minor effect. G-V curves plotted on the bottom also show lack of paxilline effect (n = 5 patches). (D) Paxilline sensitivity persists in Slo1-A313I. Currents activated with 100 µM Ca2+ are blocked by 20 and 100 nM paxilline, as also shown in the G-V curves on the bottom (n = 5 patches). (E) Paxilline blocks Slo1-M314L. Currents activated with 100 µM Ca2+ are blocked in a concentration-dependent fashion by paxilline, as summarized in the G-V curves on the bottom (n = 8 patches). Red line corresponds to fit with equivalent voltage-independent block of open and closed states with Kb = 13.3 ± 0.80 nM.