Abstract
Background
Caveolae are small, flask-like invaginations of the plasma membrane. Caveolins are structural proteins found in caveolae that have scaffolding properties to allow organization of signaling. We tested the hypothesis that delayed cardiac protection induced by volatile anesthetics is caveolae/caveolin-dependent.
Methods
An in vivo mouse model of ischemia-reperfusion injury with delayed anesthetic preconditioning was tested in wild-type, caveolin-1 knockout, and caveolin-3 knockout mice. Mice were exposed to 30 min oxygen or isoflurane and allowed to recover for 24 h. After 24 h recovery, mice underwent 30 min coronary artery occlusion, followed by 2 h of reperfusion at which time infarct size was determined. Biochemical assays were also performed in excised hearts.
Results
Infarct size as a percent of the area at risk was reduced by isoflurane in wild-type (24.0 ± 8.8% vs. 45.1 ± 10.1%) and caveolin-1 knockout mice (27.2 ± 12.5%). Caveolin-3 knockout mice did not show delayed anesthetic preconditioning (41.5 ± 5.0%). Microscopically distinct caveolae were observed in wild-type and caveolin-1 knockout mice but not caveolin-3 knockout mice. Delayed anesthetic preconditioning increased the amount of caveolin-3 protein but not caveolin-1 protein in discontinuous sucrose gradient buoyant fractions. Additionally, glucose transporter-4 was increased in buoyant fractions and caveolin-3/glucose transporter-4 colocalization was observed in wild-type and caveolin-1 knockout mice after anesthetic preconditioning.
Conclusions
These results demonstrate that delayed anesthetic preconditioning involves translocation of caveolin-3 and glucose transporter-4 to caveolae resulting in delayed protection in the myocardium.
Introduction
Exposure to volatile anesthetics prior to a lethal ischemic insult can protect the heart.1–4 This protection, termed anesthetic preconditioning (APC), has been described to be a biphasic event. Immediately after exposure to the volatile anesthetic, an early cardiac protection is observed (acute APC) which is transient and subsides after a few hours.1,2,4 Protection resumes 12–24 h after the initial stimulus (delayed APC).3 Acute APC involves the translocation and phosphorylation of pre-existing proteins, whereas, delayed APC is dependent on de novo protein synthesis.3,5–8 Acute and delayed APC involve complex signal transduction cascades.9
Signal transduction molecules are organized by scaffolding molecules into molecular complexes.10 Caveolae are small membrane invaginations on the plasma membrane that are enriched in glycosphingolipids, cholesterol, and caveolins.11,12 Three isoforms of caveolin, Cav-1, -2, and -3, are involved in the formation of caveolae and interact with signaling molecules via a scaffolding domain.13–17 All three caveolin isoforms are found in cardiac myocytes with Cav-3 being the predominant isoform.18 We have shown that both Cav-118 and Cav-319 are essential for acute APC-induced cardiac protection. We have also shown that acute ischemic preconditioning increases the formation of caveolae and that transgenic mice with cardiac myocyte-specific overexpression of Cav-3 are resistant to ischemia-reperfusion injury independent of a preconditioning stimulus.20 Thus, there appears to be a clear role for caveolins/caveolae in the regulation of acute cardiac protection from ischemia-reperfusion injury.
The initial triggering events associated with acute and delayed cardiac protection are similar. Delayed protection induces gene and protein expression changes that ultimately lead to the induction of various mediators (e.g., inducible nitric oxide synthase, 12-lipoxygenase, cyclooxygenase-2, glucose transporter-4 (GLUT-4), etc.).9,21 It is unclear how organization of mediators of delayed cardiac protection are regulated and if caveolins/caveolae play a role. As caveolae serve as a nexus for regulating acute protective signaling, we hypothesized that caveolin/caveolae would be essential for delayed APC. We show in the current manuscript that delayed APC is dependent on a specific caveolin isoform: Cav-3, and that the coordination of signaling is dependent on colocalization of Cav-3 and GLUT-4.
Materials and Methods
Antibodies
Antibodies used in this study are listed with their sources as follows: polyclonal antibody to Cav-1, Abcam (Cambridge, MA) and Cell Signaling (Danvers, MA); monoclonal and polyclonal antibody to Cav-3, BD Biosciences (San Jose, CA), Abcam, and Santa Cruz Biotechnology (Santa Cruz, CA); monoclonal antibody to GLUT-4, Abcam; monoclonal antibody to glutaraldehyde phosphate dehydrogenase, Imgenex (San Diego, CA).
Animals
All animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science, Washington, D.C.). Animal use protocols were approved by the Veterans Administration San Diego Healthcare System Institutional Animal Care and Use Committee (San Diego, California). C57BL/6 male and Cav-1 knockout mice were purchased from Jackson Laboratories (Bar Harbor, ME) and Cav-3 knockout mice were a kind gift from Drs. Ishikawa, M.D., Ph.D. (Professor, Cardiovascular Research Institute, Yokohama City University School of Medicine, Yokohama, Japan) and Hagiwara, Ph.D. (Professor, National Institute of Neuroscience, Kodaira, Tokyo, Japan) created as previously reported (8–10 weeks old, 21–26 g body weight, male).22 Animals were randomized into treatment groups by an independent observer daily. The animals were kept on a 12 h light-dark cycle in a temperature-controlled room. Mice were placed postoperatively in an animal care unit under daily supervision.
Immunoblot analysis
Protein was separated by SDS-PAGE 10% polyacrylamide precast gels (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene diflouride membrane by electroelution. Membranes were blocked in tris-buffered saline and 0.1% Tween containing 2.0% nonfat dry milk and incubated with primary antibody overnight at 4°C. Bound primary antibodies were visualized using secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology and Lumigen TMA-6 chemiluminescent reagent from GE Healthcare (Piscataway, NJ). All displayed bands migrated at the appropriate size, as determined by comparison to molecular weight standards (Santa Cruz Biotechnology).
Electron microscopy
Whole hearts were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 2 h, post-fixed in 1% OsO4 in 0.1 M cacodylate buffer (1 h) and embedded as monolayers in LX-112 (Ladd Research, Williston, VT). Sections were stained in uranyl acetate and lead citrate and observed with an electron microscope (Philips CM-10, Philips Electronic, New York City, NY). Random sections were taken by an electron microscopy technician blinded to the treatments.
Experimental preparation
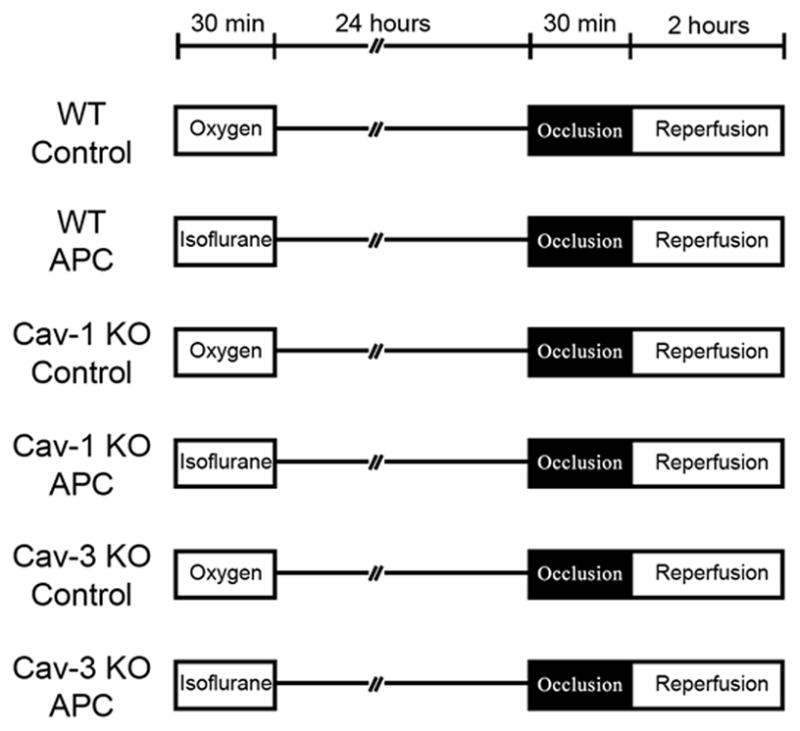
Under light anesthesia (pentobarbital sodium; 40 mg/kg intraperitoneal), mice were randomly divided into groups and received 30 min 100% oxygen in the control group or 1.4% isoflurane vol./vol. in O2 (1.0 minimum alveolar concentration for mice)23 by using a pressure-controlled ventilator (TOPO Ventilator, Kent Scientific, Torrington, CT; peak inspiratory pressure: 15 cmH2O, respiratory rate: 100 breaths/min), followed by a 24-h recovery period. Core temperature was maintained with a heating pad and lamp, and electrocardiogram leads were placed to record heart rate.
Ischemia-reperfusion protocol
After a 24-h recovery period, mice were anesthetized with pentobarbital sodium (80 mg/kg intraperitoneal) and mechanically ventilated. Hemodynamics were measured through the right carotid artery with a 1.4F Micro-tip pressure transducer (Model SPR-671, Millar Instruments, Inc., Houston, TX) as described before.24 After thoracotomy, baseline was established, and mice were assigned to one of six experimental protocols as described in figure 1. Mice then underwent 30 min index ischemia, followed by 2 h of reperfusion. After reperfusion, mice were heparinized, and the coronary artery was again occluded. The heart was immediately excised and cut into 1.0-mm slices. Each slice of left ventricle was then counterstained with 2,3,5,-triphenyltetrazolium chloride (Sigma Chemical, St. Louis, MO). After overnight storage in 10% formaldehyde, slices were weighed and visualized under a microscope equipped with a charge-coupled device camera. The images were analyzed (Image-Pro Plus version 4.5; Media Cybernetics, Silver Spring, MD), and infarct size was determined by planimetry as previously described.25
Figure 1.
Sucrose density membrane fractionation
Mice were exposed to oxygen or isoflurane as described above and allowed to recover for 24 h. Mice were anesthetized with pentobarbital sodium (80 mg/kg intraperitoneal), and hearts were excised. We used whole left ventricle to prepare sucrose density membrane fractions as reported previously.18 We defined fractions 4–6 as buoyant membrane fractions enriched in caveolae and proteins associated with caveolae. Fractions 9–12 were defined as nonbuoyant fractions.
Immunofluorescence
Ventricular tissue was mounted on a cryostat (−23 °C) and 10 μm sections were cut in the long axis. Samples were fixed with paraformaldehyde, incubated with 100 mM glycine, permeabilized in 0.1% buffered Triton X-100, blocked with 1% bovine serum albumin, phosphate buffered saline, and 0.05% Tween. Samples were then incubated with primary antibody (1:100) in 1% bovine serum albumin, phosphate buffered saline, and 0.05% Tween for 24 h. Excess antibody was removed, and samples were incubated with fluorescein Alexa-conjugated secondary antibodies (1:250) for 1 h. To remove excess secondary antibody, samples were washed with phosphate buffered saline/0.1% Tween and incubated for 20 min with the nuclear stain 4′,6-diamidino-2-phenylindole (1:5000) diluted in phosphate buffered saline. Samples were mounted in gelvatol for microscopy imaging and images were captured with DeltaVision deconvolution microscope system (Applied Precision, Inc., Issaquah, WA). The system includes a Photometrics charge-coupled device mounted on a Nikon TE-200 inverted epifluorescence microscope (Melville, NY). Three optical sections spaced 0.2 mm were taken. Exposure times were set such that the camera response was in the linear range for each fluorophore. Images were taken at 400× magnification and were deconvolved and analyzed using SoftWorx software (Applied Precision, Inc.) on a Silicon Graphics Octane workstation (SGI, Fremont, CA). Colocalization of pixels was assessed quantitatively by CoLocalizer Pro 1.0 software. All images were normalized to a background threshold value of 50.
Adult rat cardiac myocyte isolation and treatment
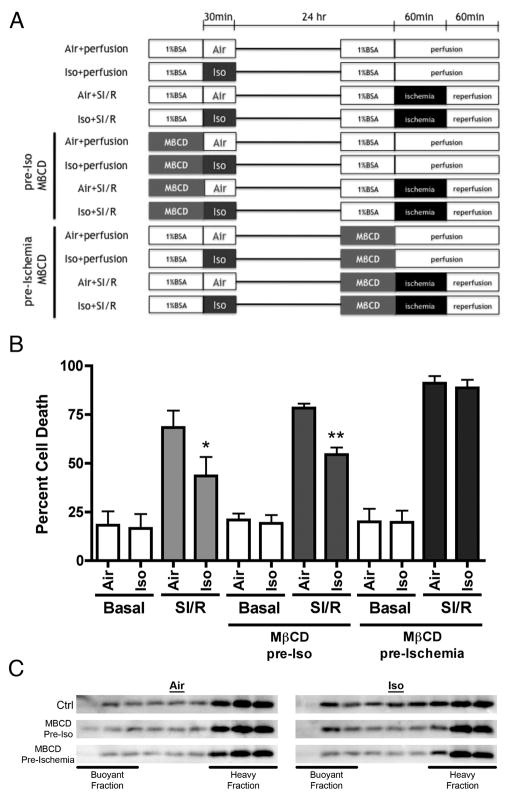
Cardiac myocytes (CMs) were isolated via retrograde-perfused Langendorff enzymatic digestion as previously described.26 Cells were plated on 12-well plates. Rat CMs were exposed to 1.4% isoflurane for 30min in a temperature controlled metabolic chamber. Chamber inflow was attached to the outflow of an isoflurane vaporizer. Chamber outflow was monitored with a Datex Capnomac capnograph (Datex). Isoflurane was infused in oxygen at 2L/min flow. We have previously confirmed that 1.4 % vol./vol. isoflurane produces 0.165±0.003mM isoflurane in media in our chamber.18 After a 24-h recovery period, the CMs were exposed to ischemic stress. Ischemia was simulated by replacing the air content with a 95% N2 and 5% CO2 gas mixture at 2L/min in a metabolic chamber and replacing the media with glucose-free DMEM media (pH 6.2) for 60 min. This was then followed by 60 min of reperfusion by replacing the media with normal maintenance media and by incubating the cells with 21% O2 and 5% CO2. Cell death was quantified by counting trypan blue stained cells with results expressed as a percentage of total cells counted. To determine the impact of intact caveolae on delayed APC-induced cardiac protection, we used methyl-β-cyclodextrin (MβCD, 1mM, 1 h), which depletes membrane cholesterol resulting in disruption of caveolae.
Statistical Analysis
Differences in hemodynamic data between groups was compared using a repeated measures two-way ANOVA with post hoc Bonferroni analysis (GraphPad Software, San Diego, CA). All other statistical analyses were performed by one-way ANOVA followed by Bonferroni post-hoc test or unpaired Student’s t-test (two-tailed). All data are expressed as mean ± SD. Statistical significance was defined as P < 0.05.
Results
Caveolin and Caveolae in Cardiac Myocytes
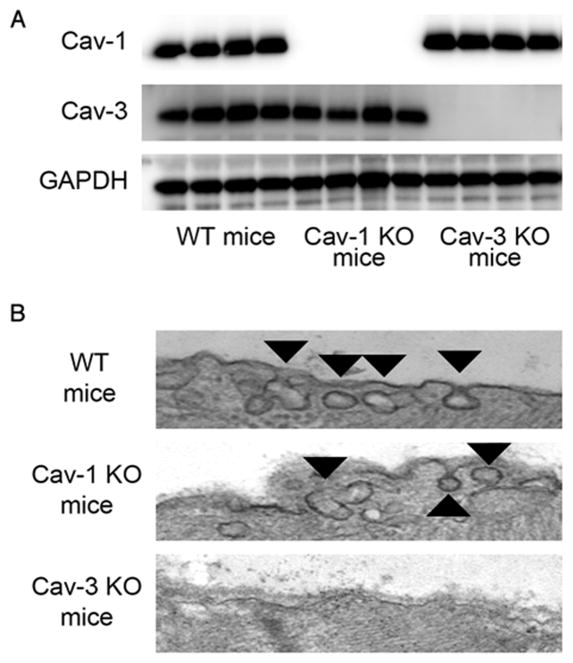
We investigated the expression of caveolin-1 and caveolin-3 protein in heart tissue. Immunoblots revealed expression of both caveolin-1 and caveolin-3 in the wild-type (WT) mouse hearts and the absence of caveolin-1 or caveolin-3 proteins in caveolin-1 knockout or caveolin-3 knockout mice, respectively (fig. 2A). Electron microscopy revealed caveolae formation in WT and caveolin-1 knockout mice, however, no caveolae were observed in caveolin-3 knockout mice (fig. 2B).
Figure 2.
Myocardial Area at Risk and Infarct Size
Mouse hemodynamics (heart rate and mean arterial pressure) following carotid artery cannulation are shown in table 1. No significant differences in heart rate or mean arterial pressure were found between groups at the pre-occlusion time point.
Table 1.
Hemodynamics
| DAY 1 |
DAY 2 |
|||||
|---|---|---|---|---|---|---|
| Baseline | 30 min treatment | Recovery | Preocclusion | Ischemia 30 min | Reperfusion 2 h | |
| Heart rate, beats · min−1 | ||||||
| WT Control | 431 ± 34 | 428 ± 23 | 425 ± 23 | 420 ± 20 | 366 ± 23* | 332 ± 18* |
| WT APC | 434 ± 45 | 413 ± 13 | 424 ± 23 | 420 ± 23 | 417 ± 30 | 379 ± 21# |
| Cav-1 KO Control | 434 ± 33 | 431 ± 30 | 424 ± 34 | 411 ± 22 | 389 ± 53 | 385 ± 26# |
| Cav-1 KO APC | 424 ± 25 | 420 ± 22 | 420 ± 15 | 417 ± 22 | 411 ± 34 | 396 ± 19 |
| Cav-3 KO Control | 442 ± 33 | 437 ± 22 | 423 ± 35 | 417 ± 29 | 381 ± 47 | 336 ± 28* |
| Cav-3 KO APC | 425 ± 14 | 438 ± 24 | 439 ± 27 | 432 ± 29 | 431 ± 30 | 410 ± 18§ |
| Mean arterial pressure, mmHg | ||||||
| WT Control | ---- | ---- | ---- | 76 ± 5 | 72 ± 5 | 61 ± 6* |
| WT APC | ---- | ---- | ---- | 77 ± 4 | 71 ± 4 | 71 ± 6 |
| Cav-1 KO Control | ---- | ---- | ---- | 76 ± 5 | 69 ± 3 | 60 ± 9* |
| Cav-1 KO APC | ---- | ---- | ---- | 75 ± 4 | 70 ± 5 | 68 ± 7 |
| Cav-3 KO Control | ---- | ---- | ---- | 75 ± 6 | 68 ± 6 | 61 ± 6 |
| Cav-3 KO APC | ---- | ---- | ---- | 78 ± 4 | 73 ± 4* | 65 ± 6* |
| Rate-Pressure Product, beats · min−1 · mmHg · 103 | ||||||
| WT Control | ---- | ---- | ---- | 32.0 ± 3.0 | 26.2 ± 3.0 | 20.3 ± 2.6* |
| WT APC | ---- | ---- | ---- | 32.1 ± 1.5 | 29.8 ± 2.9 | 26.8 ± 3.1 |
| Cav-1 KO Control | ---- | ---- | ---- | 31.2 ± 2.4 | 25.7 ± 4.4* | 23.4 ± 4.4* |
| Cav-1 KO APC | ---- | ---- | ---- | 31.5 ± 2.5 | 28.9 ± 4.2 | 27.0 ± 4.1 |
| Cav-3 KO Control | ---- | ---- | ---- | 31.5 ± 4.0 | 25.7 ± 5.5 | 20.5 ± 2.9* |
| Cav-3 KO APC | ---- | ---- | ---- | 33.8 ± 2.5 | 31.6 ± 3.6 | 26.8 ± 2.7*§ |
Data are mean ± SD. Mice were randomly exposed to 1.4% isoflurane for 30 min (DAY 1). After a 24-h recovery period, mice were subjected to 30 min of ischemia followed by 2 h of reperfusion (DAY 2).
Significantly (P < 0.05) different from pre-occlusion (intragroup comparison).
Significantly (P < 0.05) different from WT Control (intergroup comparison).
Significantly (P < 0.05) different from Cav-3 KO Control (intergroup comparison).
Abbreviations: APC: anesthetic preconditioning, Cav: Caveolin, KO: knockout, WT: wild-type.
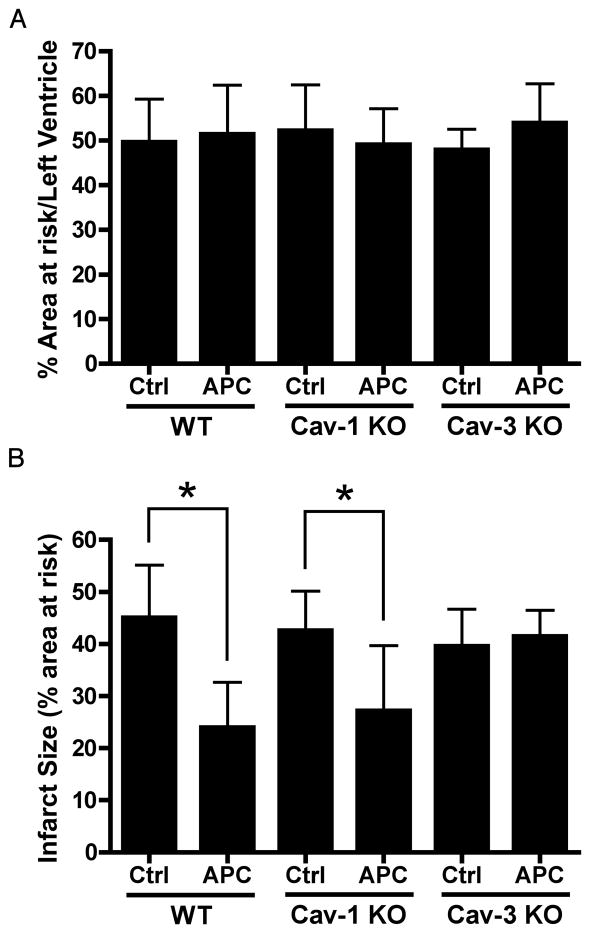
The area at risk, as a percent of the left ventricle, was similar among all groups (fig. 3A). Twenty-four hours following isoflurane (1.0 minimum alveolar concentration) exposure, a reduction in myocardial infarction was observed when compared to WT Control. In caveolin-3 knockout mice, the protection produced by isoflurane was eliminated (fig. 3B) but APC-induced cardiac protection was maintained in caveolin-1 knockout mice (fig. 3B).
Figure 3.
Isoflurane Modulates Caveolin Localization
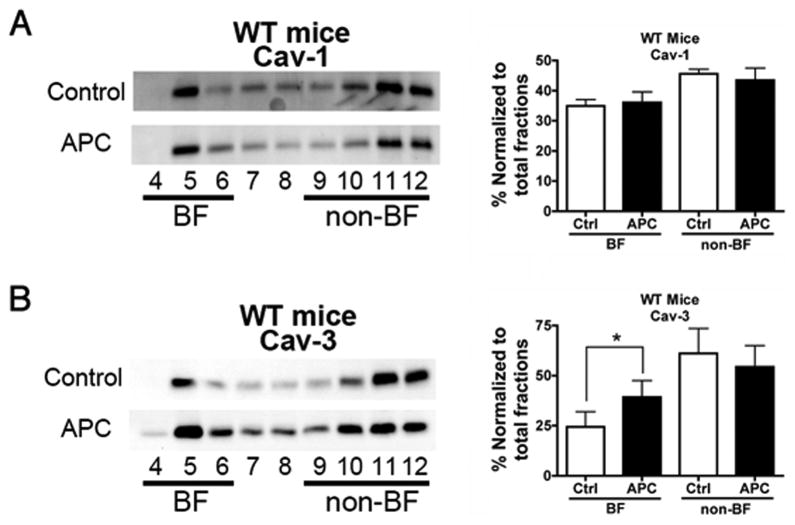
We assessed the effect of delayed APC on cardiac caveolin localization in WT mice. Hearts from Control and APC-treated animals (24 h post-APC or oxygen) were fractionated on a discontinuous sucrose gradient and analyzed for distribution of caveolin. APC increased the amount of caveolin-3 protein but not caveolin-1 into buoyant fractions (figs. 4A and B).
Figure 4.
Colocalization between GLUT-4 and Caveolins
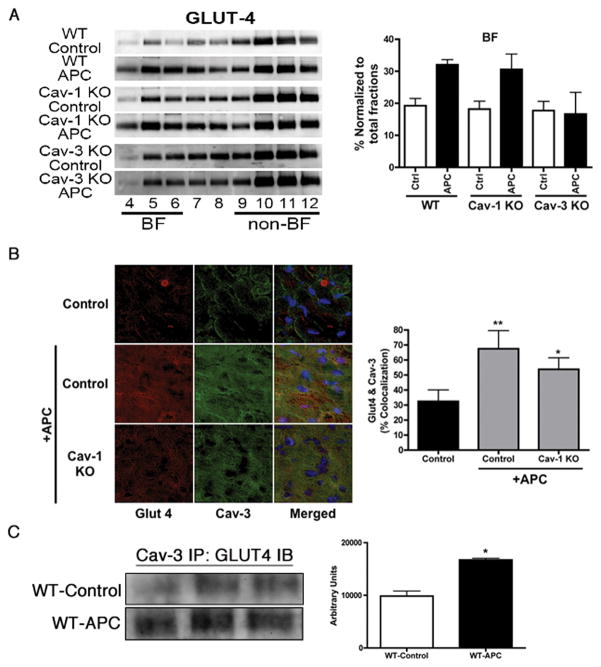
Hearts subjected to sucrose density fractionation were probed for localization of various mediators in caveolae after APC. The whole heart expression of inducible nitric oxide synthase, 12-lipoxygenase, and cyclooxygenase-2 has previously been reported to increase with delayed protective stimuli;7,27–29 the localization of these proteins was not elevated in caveolar fractions of any group (data not shown). However, higher expression of GLUT-4 was observed in buoyant fractions of WT APC and caveolin-1 knockout APC but not caveolin-3 knockout APC hearts (fig. 5A). To investigate the interaction of caveolins and GLUT-4, immunofluorescence microscopy was done. At 24-h post-isoflurane exposure (1.0 minimum alveolar concentration, 30 min), there was increased colocalization of GLUT-4/caveolin-3 in left ventricle tissues (yellow pixels, fig. 5B) in WT APC and caveolin-1 knockout APC mice. The colocalization was confirmed by immunoprecipitation with and without isoflurane treatment. Lysates were immunoprecipitated with caveolin-3 and then probed with GLUT-4 antibody. We observed increased association of caveolin-3 and GLUT-4 after isoflurane treatment (fig. 5C). The reciprocal immunoprecipitation-immunoblot was not performed as we were unable to obtain a GLUT-4 antibody suitable for immunoprecipitation.
Figure 5.
In vitro assessment of role of caveolae in delayed APC
Isolated adult rat cardiac myocytes (CM) were subject to simulated ischemia-reperfusion with and without disruption of caveolae using methyl-β-cyclodextrin (MβCD) at different times as outlined in figure 6A. Cells treated with isoflurane and allowed to recover 24 h showed significant protection when subjected to simulated ischemia-reperfusion (fig. 6B). If caveolae were disrupted with MβCD prior to isoflurane treatment, protection was still observed following 24-h recovery; however, if caveolae were disrupted with MβCD following 24-h recovery but just prior to simulated ischemia-reperfusion, the protection afforded by isoflurane was lost (fig. 6B). To further confirm disruption of caveolae, sucrose density fractionation was used to evaluate buoyant caveolin-3, an indicator of caveolae. As in the in vivo data presented in figure 4B, isoflurane treatment resulted in increased caveolin-3 localization in buoyant fractions. Treatment of CM with MβCD prior to isoflurane or air had no effect on buoyant caveolin-3; however, treatment of CM with MβCD following 24-h recovery resulted in a decline in buoyant caveolin-3 indicative of loss in caveolae (fig. 6C). These data suggest that caveolae are not necessary to trigger protection but are necessary to organize downstream signaling associated with delayed APC.
Figure 6.
Discussion
In the present study, we observed that delayed APC induced by isoflurane cannot be elicited in caveolin-3 deficient mice in vivo, indicating that the presence of caveolae (dependent on caveolin-3 expression) is a prerequisite for delayed protection in the myocardium. We further clarified the role of caveolae in in vitro studies that suggest that caveolae are not necessary for the triggering of delayed APC but may be critical to organize downstream mediators. This is the first study to investigate the role for caveolins/caveolae in delayed APC. The current study also showed that delayed APC involves translocation of caveolin-3 but not caveolin-1 into caveolae (which was also confirmed in an in vitro model of delayed APC) and this appears to be associated with specific upregulation and colocalization of GLUT-4 with caveolin-3.
Volatile anesthetics have a long history in the clinical management of anesthesia; however, recent evidence suggests a role in cardiac protection. Many studies have shown that volatile anesthetics exert biphasic cardiac protection1–3 and that characteristics of this protection are similar to those observed during classic ischemic preconditioning.30 Volatile anesthetics are short chain halogenated alkanes and ethers that interact with cell membrane lipids and directly or indirectly interact with membrane bound proteins to produce a number of cellular effects. As caveolae are highly enriched in lipids and signaling molecules, they might serve as an ideal “docking” site for volatile agents to modulate cellular physiology. To this end, we have previously shown that acute treatment of isolated cardiac myocytes with isoflurane results in increased caveolar invaginations of the sarcolemmal membrane.18 The current manuscript extends this finding to delayed protection by showing that delayed APC causes translocation of caveolin-3 and GLUT-4 to buoyant fractions and delayed APC is dependent on caveolae/caveolin-3. Previous reports have shown that lung tissue treated with sevoflurane did not show enhanced phosphorylation of caveolin-1, no data were presented with regards to modification of caveolae.31 Therefore, it is likely that different anesthetics have varied effects on caveolins and caveolae. Unpublished, preliminary data from our group suggest sevoflurane may have a similar effect to isoflurane with respect to caveolae formation but further work is necessary to validate cross class effects of volatile anesthetics on caveolins and caveolae and downstream signaling. Volatile anesthetics may preferentially interact with and modulate lipid microdomains, such as caveolae, and the proteins localized within these microdomains to regulate function. This concept may represent a novel hypothesis for a mechanism of anesthetic action that may apply to multiple organs.
We have previously shown that isoflurane-induced acute cardiac protection is abolished in caveolin-1 knockout mice despite the fact that caveolae are present in cardiac myocytes from caveolin-1 knockout mice.18 Our laboratory has also shown that caveolin-3 colocalizes with opioid receptors in vitro, which can contribute to cardiac protection from ischemia.26 More recently, we have reported that caveolin-3 knockout mice lose the ability to undergo isoflurane-induced acute cardiac protection from ischemia-reperfusion injury in both in vivo and in vitro models even though caveolin-1 is present at normal levels.19 Collectively, these data implicate caveolae and caveolins as essential to the temporal and spatial organization of acute cardiac protective signaling molecules. No previous studies have described the role for caveolae and caveolins in cardiac protection induced by delayed APC. Interestingly, we show with delayed protection that only caveolin-3 is necessary as delayed APC was absent in caveolin-3 knockout mice but robust in caveolin-1 knockout mice. These data suggest that both caveolin-3 and caveolin-1 may be important in the regulation of signaling events involved in acute cardiac protection (e.g., Src, C-terminal Src kinase, Akt, glycogen synthase kinase 3β, etc.),18,20,32 whereas, caveolin-3 and localization of components to caveolae may be critical to induction and organization of mediators involved in delayed protection.
Caveolae are cholesterol and sphingolipid enriched invaginations of the plasma membrane 50–100 nm in size33 and play a role in important physiological functions such as signal transduction,13–17 endocytosis,34 calcium homeostasis,35 and intracellular cholesterol transport.36 Proteomic studies have suggested that caveolae contain as many as 150 distinct signaling molecules;37–40 however, it has also been suggested that not all caveolae are created equal with distinct subpopulations of caveolae containing specific molecules to control specific signaling pathways.41,42 It is possible that in response to a protective stimulus certain mediators are activated and organized into caveolae to form a molecular complex that facilitates delayed protection. Certain proteins (i.e., inducible nitric oxide synthase, cyclooxygenase-2 and 12-lipoxygenase) have been shown by our group and others to be critical in mediating delayed cardiac protection in response to various stimuli where expression and activity of these proteins are upregulated in response to a stimulus and inhibition with specific pharmacologic agents abrogates protection.7,27–29 We did not observe any changes in the translocation of inducible nitric oxide synthase, cyclooxygenase-2 or 12-lipoxygenase to buoyant caveolar fractions. Such data suggest that caveolae may compartment a different subset of mediators involved in delayed protection.
We found that the caveolin-3 dependent delayed cardiac protective effect was accompanied by GLUT-4 translocation to caveolae after 24 h isoflurane exposure. Others have shown that delayed ischemic preconditioning upregulates GLUT-4 expression by activation of adenosine monophosphate-activated protein kinase in a protein kinase C-dependent manner21 and that acute ischemic preconditioning induced GLUT-4 translocation to the plasma membrane involves caveolin-3, Akt, and endothelial nitric oxide synthase.43 Therefore, GLUT-4 appears to be an important downstream mediator of delayed APC that is dependent on caveolin/caveolae for its localization.
Our data put GLUT-4 temporally and spatially downstream of other mediators that regulate delayed protection. If this is the case, one would expect GLUT-4 to be regulated by classic mediators of delayed protection. In this regard, nitric oxide donors have been shown to induce messenger RNA for GLUT-4 and translocation of GLUT-4 to the plasma membrane.44,45 Treatment of adipocytes with arachidonic acid (a precursor to cyclooxygenase-2 and 12-lipoxygenase metabolites) causes translocation of both GLUT-1 and GLUT-4 to the plasma membrane.46 Specifically, it has been shown that inhibition of cyclooxygenase can reduce the expression of GLUT-4 messenger RNA.47 Treatment of ventricular cardiac myocytes with 12-lipoxygenase inhibitors leads to altered GLUT-4 translocation from intracellular stores to plasma membrane, and this was linked to the disassembly of the actin cytoskeleton.48 It has previously been shown that caveolae and subsequent cardiac protection can be altered by cytoskeletal disruption agents.19,49,50 Such findings suggest that GLUT-4 may be a more distal mediator of delayed protection than inducible nitric oxide synthase, cyclooxygenase-2 and 12-lipoxygenase and translocation of GLUT-4 to caveolae may be a critical element of delayed APC. Our in vitro data further indicate that caveolae disruption does not alter triggering of delayed APC; however, disruption of caveolae just prior to simulated ischemia-reperfusion is sufficient to attenuate delayed APC. This suggests that caveolae are potentially important organizers of mediators induced by delayed APC.
Our findings should be interpreted within the constraints of potential limitations. We measured only heart rate and mean arterial pressure as hemodynamic parameters following isoflurane exposure. However, we showed previously that isoflurane exposure in a similar mouse protocol does not significantly alter hemodynamics or blood gases.18,25 Additionally, 24 h after isoflurane exposure, our hemodynamic data showed no differences between groups at the pre-occlusion time point. Therefore, the reductions in cardiac infarct size produced by APC most likely were not a result of changes in hemodynamic determinants. Caveolin-3 knockout mice have a variety of deleterious phenotypes (i.e., muscle degeneration,22 insulin resistance,51,52 and progressive cardiomyopathy with age53) that may impact outcome following ischemia-reperfusion injury. A recent study shows that the hearts of caveolin-3 knockout mice have normal substrate metabolism and glucose uptake.54 These contrasting data suggest that caveolin-3 knockout mice have a complex phenotype that may be organ specific. Our in vitro data suggests that caveolae are important to mediating delayed APC but not the triggering. The use of WT cells with pharmacologic disruption of caveolae get around the limitations of using caveolin-3 knockout mice and the complementary data suggest that caveolae are regulating delayed APC.
In summary, these results demonstrate that isoflurane-induced delayed preconditioning involves translocation of caveolin-3 and GLUT-4 to caveolae and that presence of microscopically distinct caveolae (dependent on caveolin-3 expression) is a requisite for delayed protection in the myocardium and specifically caveolae are necessary to mediate but not necessarily trigger delayed APC. Modulation of caveolin/caveolae may be a novel target for therapies to protect the heart from ischemia-reperfusion injury.
Acknowledgments
Funding: Supported by Beginning Grant-in-Aid 0765076Y (to Dr. Tsutsumi), Predoctoral Fellowship 06150217Y (to Mr. Horikawa), and Scientist Development Grant 060039N (to Dr. Patel) from American Heart Association, Burlingame, California; a Veterans Administration Merit Grant (to Dr. Roth) from the Department of Veterans Affairs, Washington, D.C.; and National Institutes of Health grants HL081400 (to Dr. Roth) and HL091071 (to Dr. H. Patel) from the United States Public Health Service, Bethesda, Maryland.
References
- 1.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of KATP channels: Reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: Previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997;87:1182–90. doi: 10.1097/00000542-199711000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Tonkovic-Capin M, Gross GJ, Bosnjak ZJ, Tweddell JS, Fitzpatrick CM, Baker JE. Delayed cardioprotection by isoflurane: Role of K(ATP) channels. Am J Physiol Heart Circ Physiol. 2002;283:H61–8. doi: 10.1152/ajpheart.01040.2001. [DOI] [PubMed] [Google Scholar]
- 4.Amour J, Brzezinska AK, Weihrauch D, Billstrom AR, Zielonka J, Krolikowski JG, Bienengraeber MW, Warltier DC, Pratt PF, Jr, Kersten JR. Role of heat shock protein 90 and endothelial nitric oxide synthase during early anesthetic and ischemic preconditioning. Anesthesiology. 2009;110:317–25. doi: 10.1097/ALN.0b013e3181942cb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka K, Ludwig LM, Krolikowski JG, Alcindor D, Pratt PF, Kersten JR, Pagel PS, Warltier DC. Isoflurane produces delayed preconditioning against myocardial ischemia and reperfusion injury: Role of cyclooxygenase-2. Anesthesiology. 2004;100:525–31. doi: 10.1097/00000542-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Chiari PC, Bienengraeber MW, Weihrauch D, Krolikowski JG, Kersten JR, Warltier DC, Pagel PS. Role of endothelial nitric oxide synthase as a trigger and mediator of isoflurane-induced delayed preconditioning in rabbit myocardium. Anesthesiology. 2005;103:74–83. doi: 10.1097/00000542-200507000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsumi YM, Patel HH, Huang D, Roth DM. Role of 12-lipoxygenase in volatile anesthetic-induced delayed preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H979–83. doi: 10.1152/ajpheart.00266.2006. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Lucchinetti E, Fischer G, Zhu M, Zaugg K, Schaub MC, Zaugg M. Cardiac remodelling hinders activation of cyclooxygenase-2, diminishing protection by delayed pharmacological preconditioning: Role of HIF1{alpha} and CREB. Cardiovasc Res. 2008;78:98–107. doi: 10.1093/cvr/cvn016. [DOI] [PubMed] [Google Scholar]
- 9.Weber NC, Schlack W. Inhalational anaesthetics and cardioprotection. Handb Exp Pharmacol. 2008;182:187–207. doi: 10.1007/978-3-540-74806-9_9. [DOI] [PubMed] [Google Scholar]
- 10.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–48. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Chun M, Liyanage UK, Lisanti MP, Lodish HF. Signal transduction of a G protein-coupled receptor in caveolae: Colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci U S A. 1994;91:11728–32. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–11. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 15.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273:30249–54. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers MG, Jr, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–8. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 17.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of beta -adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–57. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 18.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: A role for caveolae and caveolin-1. FASEB J. 2007;21:1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 19.Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, Ishikawa Y, Insel PA, Roth DM. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–30. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation. 2008;118:1979–88. doi: 10.1161/CIRCULATIONAHA.108.788331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–9. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara Y, Sasaoka T, Araishi K, Imamura M, Yorifuji H, Nonaka I, Ozawa E, Kikuchi T. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–54. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- 23.Deady JE, Koblin DD, Eger EI, II, Heavner JE, D’Aoust B. Anesthetic potencies and the unitary theory of narcosis. Anesth Analg. 1981;60:380–4. [PubMed] [Google Scholar]
- 24.Tsutsumi YM, Yokoyama T, Horikawa Y, Roth DM, Patel HH. Reactive oxygen species trigger ischemic and pharmacological postconditioning: In vivo and in vitro characterization. Life Sci. 2007;81:1223–7. doi: 10.1016/j.lfs.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsutsumi YM, Patel HH, Lai NC, Takahashi T, Head BP, Roth DM. Isoflurane produces sustained cardiac protection after ischemia-reperfusion injury in mice. Anesthesiology. 2006;104:495–502. doi: 10.1097/00000542-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: Role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291:H344–50. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–84. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000;97:10197–202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel HH, Fryer RM, Gross ER, Bundey RA, Hsu AK, Isbell M, Eusebi LO, Jensen RV, Gullans SR, Insel PA, Nithipatikom K, Gross GJ. 12-lipoxygenase in opioid-induced delayed cardioprotection: Gene array, mass spectrometric, and pharmacological analyses. Circ Res. 2003;92:676–82. doi: 10.1161/01.RES.0000065167.52922.F6. [DOI] [PubMed] [Google Scholar]
- 30.Baxter GF, Goma FM, Yellon DM. Characterisation of the infarct-limiting effect of delayed preconditioning: Timecourse and dose-dependency studies in rabbit myocardium. Basic Res Cardiol. 1997;92:159–67. doi: 10.1007/BF00788633. [DOI] [PubMed] [Google Scholar]
- 31.Hu G, Schwartz DE, Shajahan AN, Visintine DJ, Salem MR, Crystal GJ, Albrecht RF, Vogel SM, Minshall RD. Isoflurane, but not sevoflurane, increases transendothelial albumin permeability in the isolated rat lung: Role for enhanced phosphorylation of caveolin-1. Anesthesiology. 2006;104:777–85. doi: 10.1097/00000542-200604000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Cao H, Sanguinetti AR, Mastick CC. Oxidative stress activates both Src-kinases and their negative regulator Csk and induces phosphorylation of two targeting proteins for Csk: Caveolin-1 and paxillin. Exp Cell Res. 2004;294:159–71. doi: 10.1016/j.yexcr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–58. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson RG. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993;3:69–72. doi: 10.1016/0962-8924(93)90065-9. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993;120:1147–57. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995;92:10339–43. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Fratto P, Polvani G, Vitali E, Parolari A, Mussoni L, Tremoli E. Proteomic analysis of membrane microdomains derived from both failing and non-failing human hearts. Proteomics. 2006;6:1976–88. doi: 10.1002/pmic.200500278. [DOI] [PubMed] [Google Scholar]
- 38.Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22:985–92. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 39.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–8. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon KA, Zhu M, Kwon SW, Liu P, Zhao Y, Anderson RG. Detergent-free caveolae proteome suggests an interaction with ER and mitochondria. Proteomics. 2006;6:143–52. doi: 10.1002/pmic.200500208. [DOI] [PubMed] [Google Scholar]
- 41.Mishra S, Joshi PG. Lipid raft heterogeneity: An enigma. J Neurochem. 2007;103(Suppl 1):135–42. doi: 10.1111/j.1471-4159.2007.04720.x. [DOI] [PubMed] [Google Scholar]
- 42.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Koneru S, Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Han Z, Maulik G, Das DK, Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–72. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 44.Lira VA, Soltow QA, Long JHD, Betters JL, Sellman JE, Criswell DS. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E1062–8. doi: 10.1152/ajpendo.00045.2007. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Hu X, Selvakumar P, Russell RR, III, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–41. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- 46.Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VK, O’Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2001;276:9149–57. doi: 10.1074/jbc.M009817200. [DOI] [PubMed] [Google Scholar]
- 47.Long SD, Pekala PH. Regulation of GLUT4 gene expression by arachidonic acid. Evidence for multiple pathways, one of which requires oxidation to prostaglandin E2. J Biol Chem. 1996;271:1138–44. doi: 10.1074/jbc.271.2.1138. [DOI] [PubMed] [Google Scholar]
- 48.Dransfeld O, Rakatzi I, Sasson S, Gruzman A, Schmitt M, Haussinger D, Eckel J. Eicosanoids participate in the regulation of cardiac glucose transport by contribution to a rearrangement of actin cytoskeletal elements. Biochem J. 2001;359:47–54. doi: 10.1042/0264-6021:3590047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–9. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 50.Ismaeil MS, Tkachenko I, Gamperl AK, Hickey RF, Cason BA. Mechanisms of isoflurane-induced myocardial preconditioning in rabbits. Anesthesiology. 1999;90:812–21. doi: 10.1097/00000542-199903000-00024. [DOI] [PubMed] [Google Scholar]
- 51.Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, Williams TM, Brasaemle DL, Jelicks LA, Scherer PE, Kim JK, Lisanti MP. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C1317–31. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- 52.Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, Minamisawa S, Umemura S, Hagiwara Y, Ishikawa Y. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc Natl Acad Sci U S A. 2004;101:12670–5. doi: 10.1073/pnas.0402053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodman SE, Park DS, Cohen AW, Cheung M, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAP kinase cascade. J Biol Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 54.Augustus AS, Buchanan J, Addya S, Rengo G, Pestell RG, Fortina P, Koch WJ, Bensadoun A, Abel ED, Lisanti MP. Substrate uptake and metabolism are preserved in hypertrophic caveolin-3 knockout hearts. Am J Physiol Heart Circ Physiol. 2008;295:H657–66. doi: 10.1152/ajpheart.00387.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]