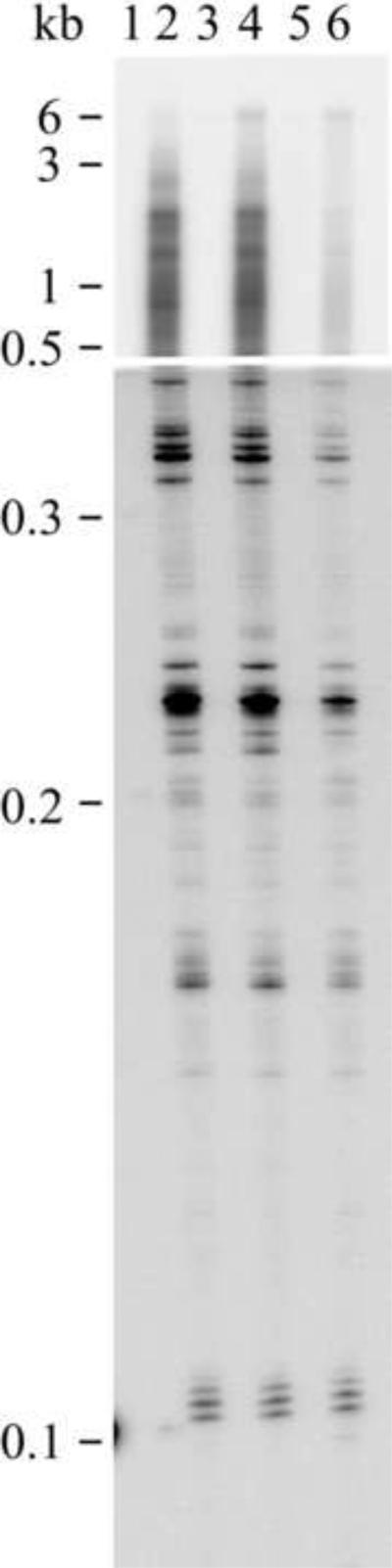
Figure 3.
Processivity of reconstituted mutant and wild-type POLG holoenzyme. Human POLG was reconstituted using a 4-fold molar excess of the accessory POLGβ subunit over POLGα, and DNA synthesis was measured at 100 mM KCl on singly-primed M13 DNA. DNA product strands were isolated, denatured and electrophoresed in denaturing 1.5% agarose (upper panel) and 6% polyacrylamide (lower panel) gels. Wild type POLGα in the absence (lane 1) and presence (lane 2) of POLGβ, lanes 3 and 4, W748S mutant of POLGα in the absence (lane 3) and presence (lane 4) of POLGβ, A467T mutant of POLGα in the absence (lane 5) and presence (lane 6) of POLGβ. Size marker: NEB 2-log DNA-ladder (molecular weights shown). As reported previously, the A467T mutant enzyme produced low-molecular-weight products similar to wildtype, but not longer products[20]. However, the W748S enzyme processivity was similar to wild type also concerning the longest products.