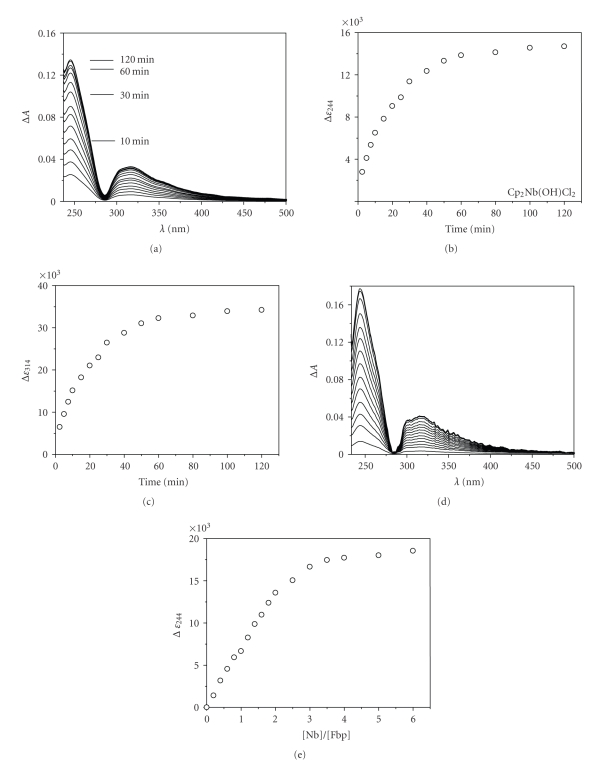
Figure 3.
(a) Difference UV/Vis spectra recorded at various times during the reaction of apoFbp (9.8 μM) with 2.0 mol equivalents of [Cp2Nb(OH)Cl2] in 10 mM Hepes buffer, 5 mM phosphate, pH 7.4 (b) Time course for reactions of apo-Fbp (ca. 9.8 μM) with 2 mol equivalents of [Cp2Nb(OH)Cl2] in 10 mM Hepes buffer, 5 mM phosphate, pH 7.4, 310 K, as a plot of molar absorptivity versus time of reaction. (c) Time course for reactions of apo-Fbp (ca. 9.8 μM) with 2 mol equivalents of [Cp2Nb(OH)Cl2] in the 10 mM Hepes buffer, 5 mM phosphate, pH 7.4 at 310 K as a plot of molar absorptivity versus time of reaction. (d) Difference UV/Vis spectra for the titration of apoFbp (9.8 μM) with [Cp2Nb(OH)Cl2] in 10 mM Hepes buffer, 5 mM phosphate, pH 7.4, 310 K (2 h equilibration). Molar ratios of Nb-complexes: apo-Fbp from bottom to top: are 0–2.0 in 0.2 mol equivalent steps, then 2.5, 3.0, 3.5, 4.0. (e) Titration curve for the reaction in (D), Δε is the absorbance at 244 nm divided by the Fbp concentration.