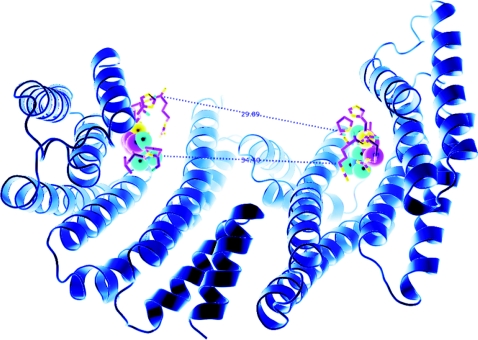
Figure 2. Structure of a 14-3-3 dimer, based on that within the AANAT/14-3-3 structure [22] in which the two phosphate-binding sites are 34.4 Å apart.
If we consider that the two binding sites are arranged in an antiparallel orientation, it is also useful to consider the distance of ~30 Å between the C-α atom of the proline of a six-amino-acid phosphopeptide [RRH(pT)LP derived from AANAT] bound to one side of the binding groove and the C-α atom of the N-terminal residue of the second peptide (arginine). This distance could be covered with a peptide containing approx. 8 amino acids in fully extended conformation, which would make 8+3+4=15 residues from one phosphorylated residue to the other (inclusive of the phosphorylated residues). Allowing (say) 4 amino acid residues for turns, would give a minimum of ~19 amino acid residues. This Figure is provided courteousy of Thomas Obsil (Charles University in Prague, Prague, Czech Republic).