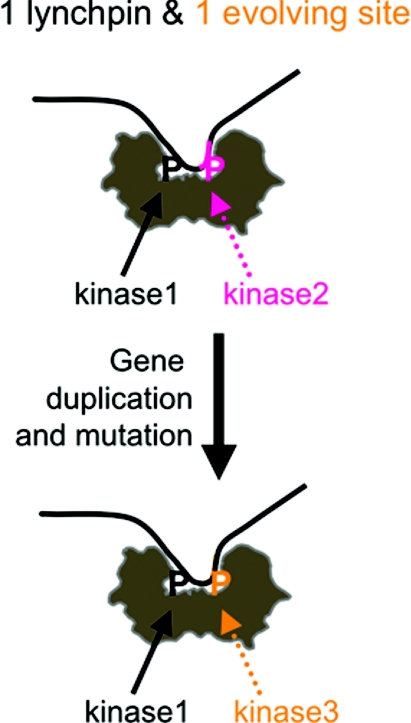
Figure 4. Hypothesis for the role of 14-3-3 dimers in driving evolutionary change of 14-3-3-binding sites in paralogous proteins.
If a copy of a 14-3-3-binding protein were to arise by gene duplication, we hypothesize that because the 14-3-3s are dimers, the function of the copy could be kept under sufficient control if one 14-3-3-binding site remained unchanged ‘the lynchpin site’, while allowing sequence divergence to occur at the second 14-3-3-binding site. If a useful consensus for phosphorylation by a different kinase were to arise at the evolving site, the result would be the generation of a paralogue with distinct regulatory inputs from the original. Whether or not 14-3-3s have actually driven evolution in this way, this theory should stimulate experimentation to compare the regulatory details of 14-3-3 binding to paralogous members of multi-protein families.