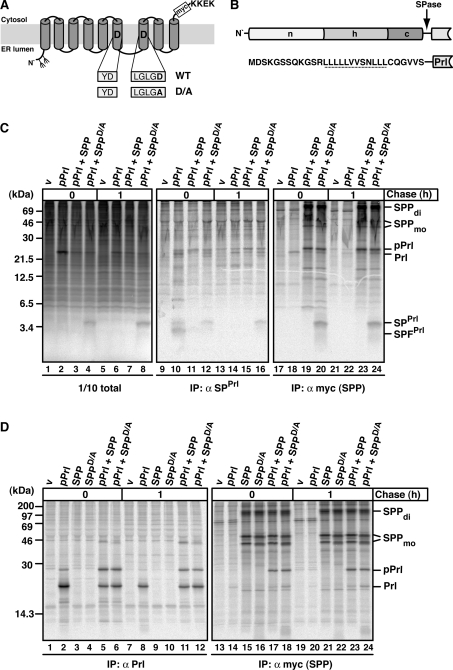
Figure 1. The effect of SPP and SPPD/A on SPPrl and pPrl processing and binding.
(A) Schematic representation of SPP in the ER membrane with amino acids of the active-site motif in one-letter code. The D→A mutation in SPPD/A and the position of the myc tag are indicated. KKEK, ER retrieval motif. Fork-like structures indicate N-linked glycosylation sites. (B) Outline and amino acid sequence of the signal sequence of pPrl with its N-terminal (n), hydrophobic (h) and C-terminal (c) region. The cleavage site for signal peptidase (SPase) is indicated, and the h-region is underlined. (C) SPPrl is trapped by the dominant-negative mutant SPPD/A. HEK-293 cells transiently expressing pPrl, SPP–myc or SPPD/A–myc were metabolically pulse labelled for 30 min and chased for 1 h where indicated. Cells were lysed in the presence of 1% Triton X-100. A 1/10 sample of the total lysate was analysed directly using SDS/PAGE and the rest was used for immunoprecipitation (IP) using either anti-SPPrl antibodies or anti-myc antibodies to identify myc-tagged SPP/SPPD/A. Samples were subjected to Tris/Tricine SDS/PAGE and labelled proteins were visualized by autoradiography. v, empty vector control; SPFPrl, signal peptide fragment of SPPrl; Prl, mature prolactin. SPP–myc dimer (di) and monomer (mo) are indicated. Note that the monomer is detected as a double-band, representing non-glycosylated and glycosylated SPP (results not shown). Molecular masses are indicated in kDa. (D) pPrl co-immunoprecipitates with SPP and SPPD/A. HEK-293 cells were transfected and pulse–chase labelled as described in (C). To detect pPrl and mature prolactin (Prl), anti-Prl antibodies were used for immunoprecipitation (IP). Anti-myc antibodies were used for the identification of myc-tagged SPP/SPPD/A and for co-immunoprecipitation. Proteins were separated by Tris/glycine SDS/PAGE and visualized as described in (C). Molecular masses are indicated in kDa.