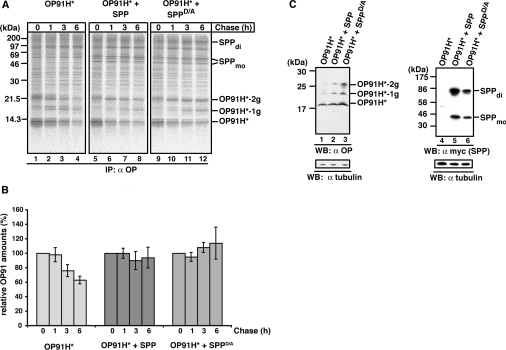
Figure 4. SPP impedes the degradation of misfolded opsin.
(A) Pulse-chase analysis of OP91H*. HEK-293 cells transiently expressing OP91H* in the absence or presence of SPP–myc or SPPD/A–myc were pulse labelled for 10 min and chased for the indicated times. Digitonin lysates were used for immunoprecipitation (IP) with the anti-opsin antibody. -1g and -2g indicate mono- and bi-glycosylated opsin forms respectively. SPP dimer (di) and monomer (mo) are indicated. Molecular masses are indicated in kDa. (B) Quantification of OP91H* accumulation. Three independent experiments as shown in (A) were used for the quantification of OP91H* accumulation in the absence (left-hand panel) or in presence of SPP–myc (middle panel) or SPPD/A–myc (right-hand panel) respectively. The OP91H* amount after the pulse (0 h chase) was set to 100%. Results are relative means±S.D. for three independent experiments. (C) Western blotting (WB) shows OP91H* accumulation in the presence of SPP. HEK-293 cells transiently expressing OP91H* and SPP–myc or SPPD/A–myc as indicated were lysed with 1% digitonin, and aliquots were separated by Tris/glycine SDS/PAGE. OP91H* and SPP/SPPD/A–myc proteins were identified using Western blotting using anti-opsin (left-hand panel) and anti-myc antibodies (right-hand panel) respectively. Membranes were stripped and re-probed with anti-tubulin antibodies as a loading control (lower panels). -1g and -2g indicate mono- and bi-glycosylated opsin forms respectively. SPP dimer (di) and monomer (mo) are indicated. Molecular masses are indicated in kDa.