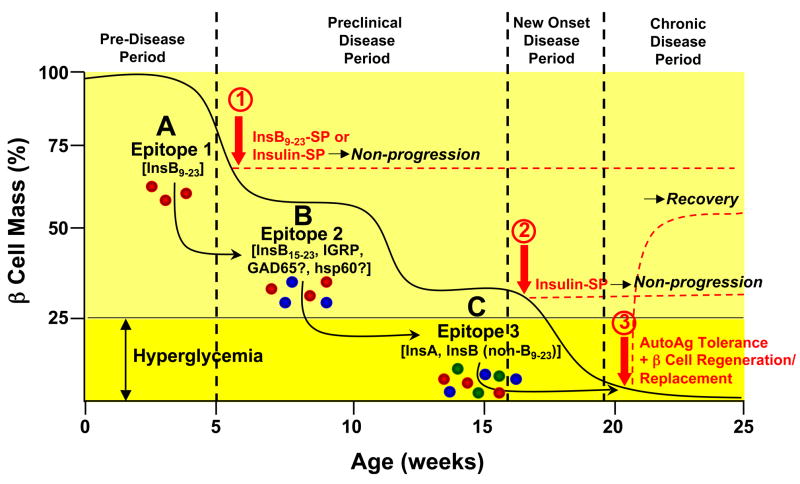
Figure 1. Model of Epitope Spreading and Tolerance Therapy in the Pathogenesis of Type 1 Diabetes in the NOD Mouse.
Progression of T1D in the NOD mouse involves the sequential activation of autoreactive T cells to multiple diabetogenic epitopes via epitope spreading which accumulate until clinical diagnosis when sufficient autoreactive effector cells are present to cause destruction of the majority of the β cell mass. The insulin B chain epitope 9–23 (InsB9–23) (A - red effector cells) appears to be the initiating or very early pathogenic diabetogenic epitope in the NOD mouse based on the ability of tolerance induced by ECDI-fixed splenocytes coupled with either intact insulin or InsB9–23 in 4–6 week old mice to inhibit development of clinical diabetes (1). As β cell destruction continues responses to additional islet antigens, e.g. InsB15–23 and/or IRGP (B - blue effector cells) and eventually epitopes on the insulin A or B chains (C - green effector cells) are activated. Epitopes on the InsA or Ins B chain (outside of B9–23) epitopes on chain appear to be dominant at the stage of transition to overt disease (loss of approximately 75% of islet mass) based on the ability tolerance induced by insulin-coupled, but not InsB9–23-coupled, splenocytes to ameliorate disease progression in 18–20 week old NOD mice (2). Recovery from (i.e., reversal) chronic T1D when all of the β cells have been destroyed would be expected to require a combination of tolerance to the autoantigens which were responsible for initial β cell destruction and a β cell regeneration and/or replacement strategy which may require allo- or xenoantigen tolerance in therapies involving islet cell transplantation (3). A similar pattern of epitope spreading is postulated to occur in human T1D.