Table 2.
13C NMR Data of Compounds 1-2c
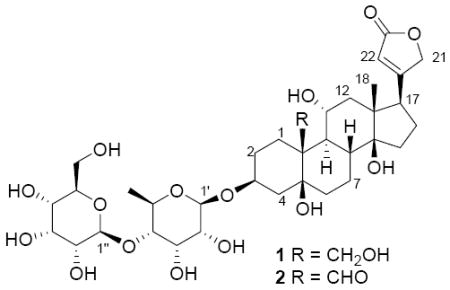 | |||
|---|---|---|---|
| no. | 1a | 1b | 2b |
| 1 | 22.7 | 22.4 | 21.3 |
| 2 | 27.6 | 27.7 | 27.1 |
| 3 | 74.8 | 76.2 | 75.5 |
| 4 | 35.3 | 35.9 | 35.7 |
| 5 | 76.3 | 77.5 | 75.5 |
| 6 | 37.3 | 37.0 | 38.7 |
| 7 | 25.0 | 25.1 | 25.5 |
| 8 | 40.8 | 41.1 | 42.2 |
| 9 | 45.1 | 45.2 | 46.1 |
| 10 | 45.4 | 45.7 | 56.7 |
| 11 | 68.7 | 69.1 | 68.5 |
| 12 | 50.7 | 50.6 | 50.1 |
| 13 | 51.0 | 51.8 | 51.0 |
| 14 | 85.0 | 85.9 | 85.4 |
| 15 | 33.5 | 33.5 | 33.1 |
| 16 | 28.0 | 28.0 | 28.0 |
| 17 | 51.4 | 51.1 | 51.5 |
| 18 | 18.2 | 17.8 | 17.6 |
| 19 | 65.6 | 65.9 | 211.1 |
| 20 | 174.8 | 177.2 | 177.2 |
| 21 | 74.0 | 75.5 | 75.5 |
| 22 | 118.1 | 118.1 | 118.1 |
| 23 | 175.8 | 177.7 | 177.5 |
| 1′ | 99.3 | 99.5 | 99.5 |
| 2′ | 65∼80d | 72.3 | 72.4 |
| 3′ | 65∼80d | 72.2 | 72.2 |
| 4′ | 83.8 | 84.0 | 84.0 |
| 5′ | 65∼80d | 69.9 | 69.9 |
| 6′ | 18.7 | 18.3 | 18.3 |
| 1″ | 104.3 | 103.7 | 103.7 |
| 2″ | 65∼80d | 72.4 | 72.4 |
| 3″ | 65∼80d | 73.3 | 73.3 |
| 4″ | 65∼80d | 68.6 | 68.6 |
| 5″ | 65∼80d | 75.4 | 75.4 |
| 6″ | 63.0 | 62.8 | 62.8 |
in pyridine-d5.
in CD3OD.
δ (ppm) 100 MHz.
resonances not assigned because of overlapping
1H NMR resonances in the HMBC spectrum.
