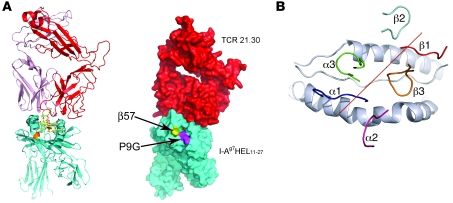
Figure 4. Overview of the TCR 21.30/I-Ag7HEL11–27 complex.
(A) Ribbon trace of the complex (left). The TCR 21.30 α and β chains are colored pink and red respectively. I-Ag7 is colored turquoise, except for residue β57 (orange). The HEL peptide is shown as ball-and-stick. Right panels shows the same view, with a space-filling representation showing that both β57 and the P9 pocket lie outside of the TCR footprint. (B) Diagonal orientation and footprint of the 6 CDRs of TCR 21.30 on pMHC. The red line denotes the relative diagonal orientation of the TCR to pMHC and is the linear fit to the centers of gravity of the conserved VαVβ sulfurs. The I-Ag7 α helices, which form the walls of the peptide-binding groove, are drawn as cartoon representations and are colored silver.