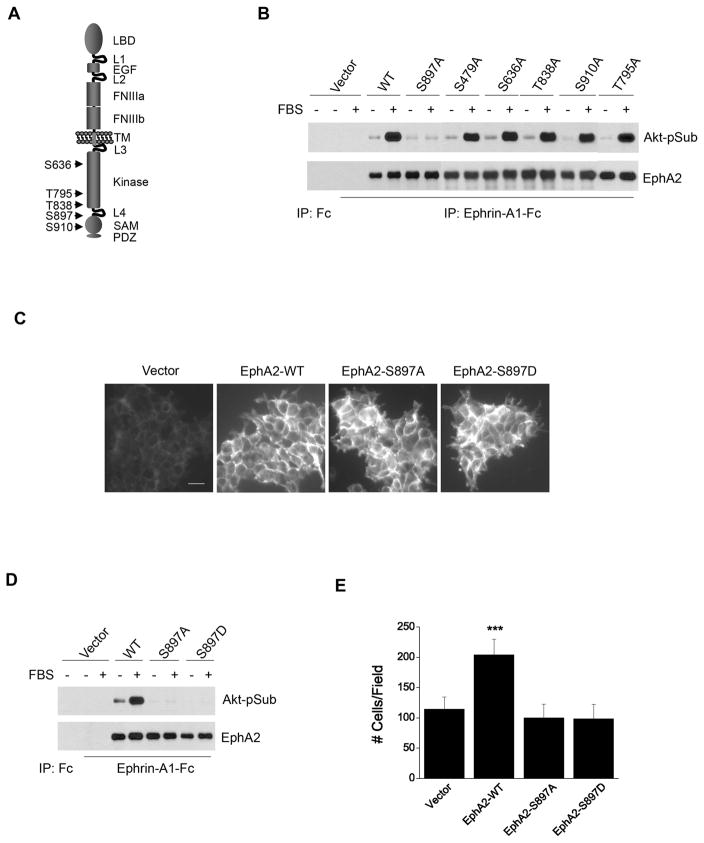
Figure 2. Serine 897 of EphA2 is the major substrate site for Akt activity, which is essential for ligand-independent promotion of cell migration.
A. Schematic illustration of relative positions of putative Akt substrate sites in EphA2 cytoplasmic tail.
B. S897A mutation abolishes serum-induced S/T phosphorylation of EphA2. HEK 293 cells were infected with retroviral vectors expressing WT or mutant EphA2. EphA kinases were precipitated and immunoblotted for Akt-pSub and total EphA2.
C. Immunofluorescence staining of infected HEK 293 cells shows that the exogenous EphA2 receptors were homogeneously expressed. Scale bar, 25 μm.
D. Both S897A and S897D mutations completely abolish the serum-induced phosphorylation of EphA2 on Akt substrate sites. Cells were stimulated and analyzed as described in B.
E. Overexpression of WT-EphA2 but not S897A-EphA2 or S897D-EphA2 enhances serum-stimulated cell migration. Cell migration assay was performed as described in Fig. 1. Numbers represent mean ± S.D. ***, p<0.001.