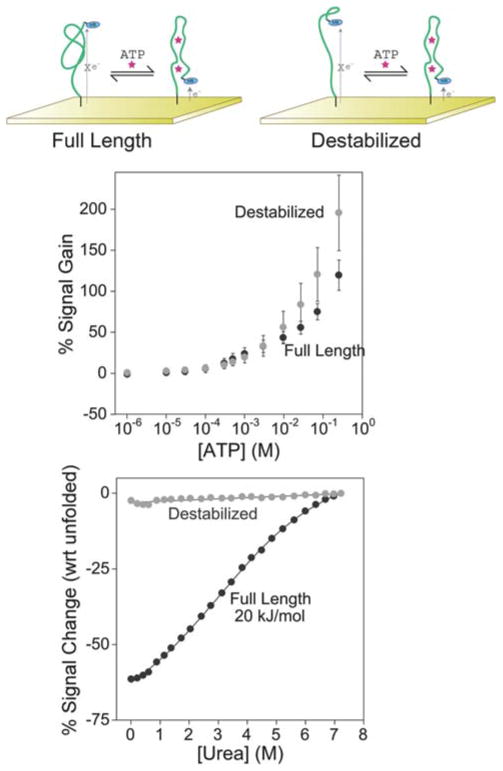
Fig. 2.
Both the full-length anti-ATP aptamer (top left) and a destabilized sequence (top right) support efficient E-AB signaling (middle). The destabilized sequence produces a gain (relative signal change in the presence of target) of 190%, which is slightly greater than the 130% observed for the full-length aptamer. Urea denaturation curves (bottom) performed on electrode-bound aptamers indicate that the full-length sequence is folded in the absence of ATP. The destabilized construct, in contrast, appears to be unstructured in the absence of target. Reported error bars reported here in and in the following figures represent the standard deviation of measurements taken from at least three independently fabricated sensors.