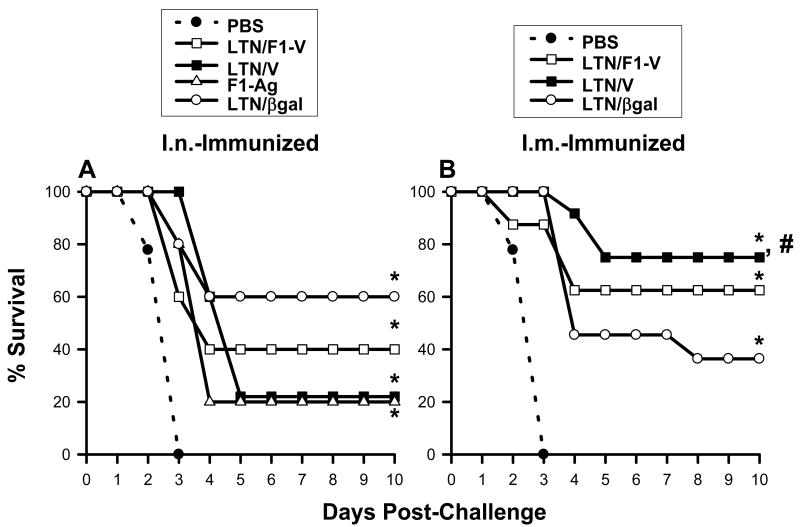
Fig. 4.
LTN DNA vaccines confer effective protection against pneumonic plague. (A) Mice were nasally dosed on wks 0, 1, 2, and 12 with LTN/βgal (n = 5), LTN/F1-V (n = 5), or LTN/V (n = 5) DNA and boosted on wks 8, 9, and 12 with recombinant F1-Ag plus CT adjuvant. An additional group received recombinant F1-Ag (n = 5) plus CT adjuvant only on wks 8, 9, and 12; a negative control group received PBS only (n = 14). All mice were challenged 2-6 weeks after the last immunization. Survival fractions obtained from vaccinated-mice were compared to PBS-dosed mice, and significance was determined: *P < 0.005. (B) Similar immunization regimen using LTN DNA vaccine plus recombinant F1-Ag, as described, “A” was applied to i.m.-dosed mice using the LTN/βgal (n = 11), LTN/V-Ag (n = 12) or LTN/F1-V (n = 8) DNA vaccines. A negative control group received PBS only (n = 14). Survival fractions obtained from vaccinated mice were compared to PBS-dosed mice as well as between routes of immunization, and significance was determined: *P < 0.001 and #P < 0.05, respectively.