Abstract
The aim of this study was to examine aortic biopsies with a cytokine array to identify new cytokines associated with abdominal aortic aneurysm (AAA). We assessed the relative expression of 79 cytokines using antibody-based cytokine arrays in a total of 12 AAA and 12 control aortic biopsies. Based on these findings we validated the findings for one cytokine by examining a further 11 AAA and 11 atherothrombosis biopsies and serum from 1028 men, 315 of whom had an AAA. Three cytokines (interleukins 1B and 8, and Chemokine CC motif ligand 22 [CCL22]) were consistently up-regulated in AAA biopsies. Since CCL22 had not previously been associated with aortic dilatation, we confirmed the upregulation of this cytokine in further tissue biopsies and serum using enzyme-linked immunosorbent assay. Median serum concentrations of CCL22 were greater in men with AAA (0.69 ng/ml) than controls (0.56 ng/ml, P < 0.01). Serum CCL22 was independently associated with both small (OR 1.51, 95% CI 1.21–1.88) and large AAA (OR 1.33, 95% CI 1.08–1.62) after adjusting for other risk factors. The association between CCL22 and AAA was also confirmed using immunohistochemistry. The results presented in this study demonstrate a novel association between CCL22 and AAA as well as illustrate how a protein array can be used to identify novel markers of potential pathogenic and diagnostic significance for AAA.
Abdominal aortic aneurysm (AAA) is recognized as an important cause of mortality.1 Marked accumulation of leukocytes, macrophages, B and T cells is a consistent finding in biopsies of human AAA.2,3 The influx, migration, and effects of these cells are controlled by an array of pro-inflammatory cytokines, chemokines, and growth factors (referred to collectively as cytokines in this article).4,5 High concentrations of various cytokines have been demonstrated in both AAA biopsies and within the circulation of patients with AAA.6,7,8 Some cytokines, such as interleukin (IL) 6, have been shown to be present within the circulation at increased concentrations distal to AAAs, suggesting they are released directly from the aortic wall.8 At present, however, which cytokines are most relevant to AAA is not clear.
We hypothesized that cytokines up-regulated within human AAA biopsies would also be present in increased concentrations within the circulation of patients. The aim of this study was to identify cytokines consistently up-regulated within human AAA biopsies using antibody arrays to measure relative expression of 79 proteins. The up-regulation of one cytokine in human AAA, not previously associated with aortic dilatation, was validated in additional aortic biopsies using alternative outcome techniques and further examined within the blood of 1028 men to assess any association between circulating levels and AAA.
Materials and Methods
Study Design and Patients
We initially performed three studies to identify and validate cytokines differentially expressed in AAAs using a total of 46 biopsies from 23 patients with AAAs and 15 patients with atherothrombosis. In study 1, we compared the relative concentrations of 79 different cytokines (listed in Supplemental Table S1 at http://ajp.amjpathol.org) in biopsies from the center of AAAs (n = 4) to those from patients with aortic atherosclerosis (n = 4). In study 2, we compared the relative concentrations of the same 79 cytokines in biopsies removed from the body (n = 8) and the nonaneurysmal neck of AAAs (n = 8). In study 3 we attempted to validate the findings for one cytokine in more detail using the alternative measurement technique of enzyme-linked immunosorbent assay (ELISA). We compared the concentration of this cytokine in biopsies from the wall of AAAs (n = 11) and those from atheroma removed from patients with atherothrombosis (n = 11). Finally, in study 4, we assessed the association between circulating levels of two cytokines and AAA presence (n = 1028). No patient was included in more than one study. Patients were recruited from Queensland for the aortic biopsy investigations (studies 1, 2 and 3). Inclusion criteria included age between 65 and 80 years, absence of diabetes and treatment by angiotensin converting enzyme inhibitor or angiotensin receptor blockers. The latter patients were excluded due to the potential effect of diabetes and angiotensin inhibition on cytokine concentrations.9,10 All patients were receiving aspirin and a statin, in line with current recommendation.11 For study 4, we used subjects from the Health In Men Study, which has previously been described in detail.12 From this cohort of men we assessed samples from all men with AAAs with available blood samples (n = 315 of which aortic diameter distribution was 245 with 30 to 39 mm, 53 with 40 to 49 mm, and 17 with ≥50 mm) and randomly selected a further 713 men without AAAs as controls. AAA was defined as maximum infrarenal aortic diameter ≥30 mm assessed by ultrasound (study 4) or computed tomography (studies 1, 2, and 3), as in previous studies in which we have reported reproducibility of these techniques.13 Ethics approval was provided for these studies from the relevant committees. All patients provided written consent to their inclusion.
Aortic Biopsies
Full thickness biopsies were taken from the anterior wall of the infrarenal aorta near its center opposite the inferior mesenteric artery in patients undergoing repair of AAA (n = 23) or bypass for atherothrombosis (n = 4). Adherent thrombus was carefully removed from the luminal side of the samples. In eight patients additional biopsies were also taken from the AAA at the site of the proximal graft anastomosis where the aortic diameter was relatively normal (study 2). Aortic biopsies were initially stored at −80°C before batch analysis. Biopsies were ground under liquid nitrogen, and proteins were extracted and quantified as previously described.14
Peripheral Atheroma Biopsies
To more precisely assess the location of one cytokine and to assess the validity of our findings from the cytokine array we analyzed the concentration of CCL22 in atheroma biopsies removed as part of a femoral (n = 5) or carotid endaraterectomy (n = 6). The atheroma biopsies were taken from the macroscopically diseased site of the endarterectomy sample and processed in the same manner as the aortic biopsies.
Cytokine Arrays
Human cytokine antibody arrays for tissue lysates were obtained commercially (Millipore, AA2005H-8) and assayed as per manufacturer’s instructions. Array membranes consisted of immobilized antibodies to 79 cytokines (see Supplemental Table S1 at http://ajp.amjpathol.org). The arrays for each comparison (study 1: 4 AAAs versus aortic atherothrombosis; study 2: 8 body versus neck of AAAs) were developed together. Briefly, array membranes where incubated for 2 hours at room temperature with 50 μg of protein extracted from relevant aortic biopsies. Membranes were washed and incubated a further 2 hours with a cocktail of biotin-conjugated, anti-cytokine, primary antibodies before a 1-hour incubation in the presence of horseradish peroxidase (HRP)-conjugated streptavidin. Cytokine signal (array spot) was visualized using enhanced chemiluminescence (ECL Advance; Amersham Biosciences) and assessed using the ChemiDoc imaging system (Bio-Rad Laboratories) and QuantityOne 1-D Analysis Software (Bio-Rad Laboratories). Relative concentration of cytokines was quantified by spot volume taking into account area, density, and normalization, and then referred to by convention as relative density units.14
Histology and Immunohistochemistry
A random selection of AAA (n = 4) and atheroma (n = 4) biopsies were examined by histology and immunohistochemistry. Eight-micron cryostat sections fixed with 75% chilled methanol in PBS for 10 minutes were stained using Verhoeff’s elastin method and counterstained with van Geison’s stain. Briefly hydrated sections were stained for 15 minutes in Verhoeff’s solution then differentiated with 2% ferric chloride. Counterstaining was performed with van Geison’s stain for 2.5 minutes then sections were dehydrated and mounted as per standard methods. Immunohistochemistry was performed using the Rennaissance TSA biotin amplification system (Perkin Elmer, NEL700001KT) and chromogenic diaminobenzidine substrate (Immpact, Vector labs, SK-4105). The primary antibodies (Abs) used were from a variety of sources: Control mouse Abs (BD Pharmingen), conIgG1-fluorescein isothiocyanate (FITC) (1/50, Dako, X0927), CD68-FITC (1/50, Dako, F7135), neutrophil elastase (1.1 μg/ml, Dako, M0752), mast cell tryptase (0.85 μg/ml, Dako, M7052), goat IgG (8 μg/ml, Vector labs, I-5000), goat anti-CCL22 (8 μg/ml, Santa Cruz, SC-12285), and goat anti-CCR4 (8 μg/ml, Santa Cruz, SC-6126). Two different secondary Ab systems were used. For the unlabeled primary Abs, the multilink-biotin/streptavidin-HRP system was used (both at 1/500, Dako, E0453/P0397) for the FITC labeled primary Ab, the secondary used was HRP-conjugated rabbit anti-FITC Ab (1/1000, Invitrogen, A-212253). Several steps were used to minimize background staining; an initial 30-minute 0.3% hydrogen peroxide incubation to block endogenous peroxidase activity, a 15-minute 10% normal serum block matched to the secondary Ab, 15-minute incubation to block endogenous activity due to avidin and biotin (Vector labs, SP-2001), and inclusion of 2% normal serum matched to the secondary in the secondary Ab incubation step. The sections were also counterstained with hematoxylin.
Blood Assays
Chemokine CC motif ligand 22 (CCL22) and interleukin (IL)-6 were assessed in serum and plasma, respectively, using ELISA according to manufacturer’s instructions (R&D Systems). Detection limits for these assays were 7.8 and 1.1 pg/ml. We included eight repeat samples between assay days to assess errors. Intra- and interassay coefficients of variations were between 3 and 10% and concordance correlation coefficients between 0.91 and 0.99. C-reactive protein was measured by a high-sensitivity assay, with the use of the particle-enhanced immunonephelometry system on the BNII analyser.
Assessment of Biopsies in Study 3
Protein was extracted from the biopsies as for the cytokine array and CCL22 measured by ELISA (R&D Systems).
Analysis of the Results of the Cytokine Arrays
The arrays incorporated a number of technical controls. Each membrane contained three negative controls designed to set the minimum intensity to differentiate from background staining. Each membrane also contained six positive controls designed to develop within the middle of the dynamic range of the test and therefore allow comparison between arrays. These controls were used to normalize array results thereby allowing comparison between membranes. First the mean intensity for negative controls (n = 3) were calculated for each membrane and any spots with readings below this reading were designated as zero. Next the mean intensity for positive controls (n = 6) were calculated for each membrane and used to normalize between membranes. Relative aortic cytokine concentrations after normalization were expressed as relative density units per μg of protein. Findings were compared between AAA and athero-thrombosis biopsies or between body and neck of AAA samples using Mann Whitney U-test and Wilcoxon signed rank test, respectively. Results were corrected for multiple testing to generate false discovery rate adjusted P values, as previously described.15,16 Cytokines were considered to be consistently significantly differentially expressed if they were similarly measured at increased or decreased concentrations relative to control biopsies in both studies 1 and 2 with corrected P values of ≤0.05.
Analyses of Studies 3 and 4
The cytokine concentrations measured in biopsies of AAA and atherothrombosis by ELISA in study 3 were compared with Mann Whitney U-test. In study 4, characteristics of patients with and without AAA were initially compared using χ2 for nominal variables and Kruskal Wallis test for continuous variables. To compare serum cytokines between patients with and without AAA multiple logistic regression was used to adjust for other risk factors (age, hypertension, coronary heart disease, dyslipidemia, smoking, diabetes, aspirin prescription, serum creatinine, C-reactive protein, and waist-hip ratio). These risk factors were adjusted for since they have previously been associated with AAA or found to influence circulating cytokine concentrations. The association of circulating cytokines with AAA was further investigated using receiver operating characteristic curves and area under the curve. We used two-sided statistical tests.
Results
Cytokines Differentially Expressed in AAA and Control Biopsies Defined by Antibody Arrays
The characteristics of the patients included in the aortic cytokines antibody array investigations (studies 1 and 2) are shown in Table 1. The patients and controls were carefully matched for age, risk factors, and medication history, as explained in Methods and Materials, and by virtue of using control biopsies from the AAA neck in study 2. Examples of the histological characteristics of the biopsies are shown in Figure 1, A–F. A total of 15 cytokines were differentially expressed between biopsies of four AAAs and four atherothrombotic aortas in study 1. Seven cytokines were present at greater concentrations within AAA biopsies (tumor necrosis factor [TNF] receptor superfamily [TNFRSF]11B, IL1B, IL5, IL8, IL10, TNFα, CCL22), while eight were more highly expressed in samples of atherothrombosis (CCL4, CCL5, CCL26, chemokine C-X-C ligand [CXCL]9, interferon [INF]γ, angiogenin, neurotrophin 3, insulin-like growth factor [IGF]BP2; see Supplemental Table S1 for details at http://ajp.amjpathol.org). In study 2, 18 cytokines were up-regulated within the body compared with the non-dilated neck of a further eight AAAs (CCL2, CCL4, CCL5, CCL22, CXCL2, CXCL5, IL1A, IL1B, IL3, IL8, INFγ, IGF1, transforming growth factor [TGF]B2, TNFβ, glial cell line derived neurotrophic factor [GDNF], KIT ligand [KITLG], creatine kinase (CK), tissue inhibitor of metalloproteinase [TIMP]1; Supplemental Table S1 at http://ajp.amjpathol.org). Three cytokines, namely IL1B, CCL22, and IL8, were consistently differentially expressed in the 12 AAA biopsies relative to the 12 control biopsies in both studies (Table 2). These cytokines were significantly up-regulated in AAA biopsies relative to both groups of control samples.
Table 1.
Comparison of Patients with and without AAA Undergoing Aortic Cytokine Measurements
| Study group | One
|
Two AAA | Three
|
||
|---|---|---|---|---|---|
| AAA | Atherothrombosis | AAA | Atherothrombosis | ||
| Number | 4 | 4 | 8 | 11 | 11 |
| Maximum aortic diameter (mm) | 60 (60–68)* | 20 (18–24)* | 60 (50–70)† | 62 (56–65)* | 23 (18–25)* |
| AAA neck diameter (mm) | NA | NA | 22 (20–26)† | NA | NA |
| Age (years) | 72 (65–79) | 69 (65–75) | 72 (67–77) | 71 (67–78) | 70 (68–74) |
| Male | 4 | 4 | 4 | 11 | 11 |
| Hypertension | 3 | 3 | 6 | 8 | 9 |
| Ever smoker | 3 | 4 | 6 | 9 | 11 |
| CHD | 3 | 3 | 5 | 5 | 6 |
Nominal variables are presented as numbers. Continuous variables are presented as median (inter-quartile range) and compared by Mann-Whitney U test. CHD = coronary heart disease; AAA = abdominal aortic aneurysm.
Maximum aortic diameter was greater in patients with AAA, as compared with those with atherothrombosis in studies 1 and 3, P = 0.03 and <0.01, respectively, by Mann-Whitney U test.
Aortic diameter was significantly greater in the body compared with the proximal neck of the AAAs included in studies 2, P < 0.01 by Mann-Whitney U test. NA = Not assessed.
Patients were only included if they were aged 65 to 80 years, were not diabetic and were receiving statins.
Figure 1.
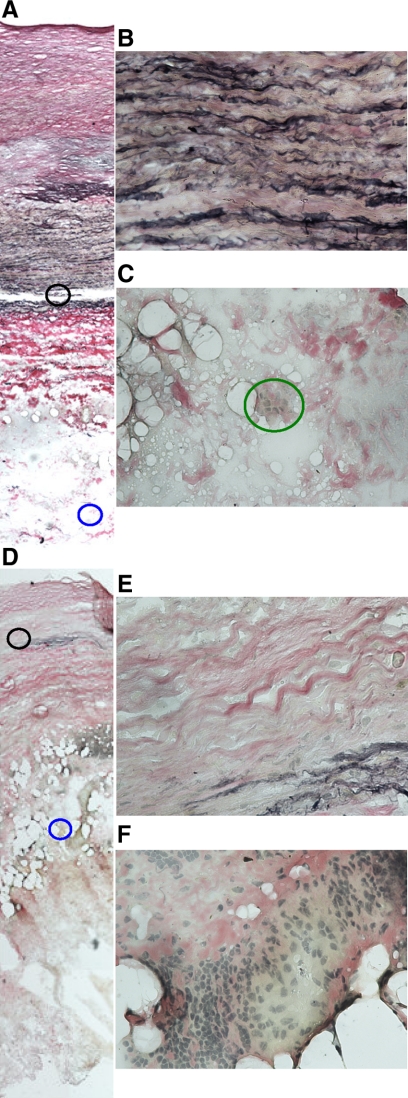
Examples of histological findings of biopsies taken from the neck (A, B, C) and body (D, E, F) of an aortic aneurysm. Sections were stained by the Verhoeff-van Gieson technique, which stains collagen red, elastin black, and nuclei gray. Photomicrographs are at ×10 (A and D) and ×40 (insets of media highlighted with black circles, B and E, and adventitia highlighted with blue circles, C and F). The section from the AAA neck biopsy illustrates a relative maintained elastic media (A and B) with minimal infiltration of the adventitia by inflammatory cells (A and C, highlighted with a green circle). In contrast the biopsy from the body of the AAA has marked medial elastin degradation (D and E) and infiltration of the adventitia by inflammatory cells (D and F).
Table 2.
Relative Concentrations of Three Cytokines Consistently Up-Regulated in Human AAAs Biopsies
| Experiment Cytokine | One
|
Two
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AAA | Atherothrombosis | Fold difference | P values* | FDR-adjusted P values | Body | Neck | Fold difference | P values† | FDR-adjusted P values | |
| CCL22 | 2.57 (2.01–3.03) | 1.40 (1.15–1.45) | 1.84 | 0.01 | 0.05 | 37.56 (36.08–39.59) | 17.24 (10.93–19.88) | 2.18 | 0.01 | 0.05 |
| IL1B | 1.93 (1.25–2.41) | 0.51 (0.05−0.92) | 3.78 | 0.01 | 0.05 | 49.80 (37.78–60.13) | 27.76 (21.09–33.39) | 1.79 | 0.01 | 0.05 |
| IL8 | 15.70 (13.53–17.79) | 7.68 (7.05–9.72) | 2.04 | 0.02 | 0.05 | 20.27 (17.79–24.90) | 9.76 (4.19–14.61) | 2.08 | 0.01 | 0.05 |
Shown are median and inter-quartile range of relative density units/ μg of protein. Only cytokines with relative concentrations consistently significantly different between AAA and atherothrombosis (study 1) and between body and neck of AAA biopsies (false discovery rate, FDR, adjusted P ≤ 0.05) are given. All other results are given in Supplementary Table S1 at http://ajp.amjpathol.org.
Statistical comparisons were made using Mann Whitney U * and Wilcoxon sign rank
tests. CCL22 = chemokine CC motif ligand 22; IL = interleukin.
Validation of Up-Regulation of CCL22 in AAA Biopsies
From the three cytokines consistently up-regulated within AAA biopsies in studies 1 and 2 we selected one for further assessment, namely CCL22, in a validation study (study 3) using alternative samples (11 AAA and 11 atherothrombosis biopsies) and outcome assessment method. This cytokine was chosen since the other two cytokines have previously been demonstrated to be up-regulated within AAA biopsies in numerous other studies.17,18,19,20,21,22 The characteristics of the patients included in study 3 are shown in Table 1. The risk factors for patients with AAA and peripheral atheroma were similar. The median concentrations of CCL22 in the biopsies of AAA and atheroma were 14.50 (n = 11, interquartile range 10.00–18.77) and 0.09 (n = 11, interquartile range 0.08−0.15) pg/mg of protein respectively, P < 0.0001. CCL22 concentrations were similar in atheroma removed from the femoral (n = 5, median 0.08 pg/mg of protein, interquartile range 0.01−0.11) and carotid arteries (n = 6, median 0.12 pg/mg of protein, interquartile range 0.08−0.25), P = 0.13.
Distribution of CCL22 and Its Receptor in AAA Biopsies
The expression of CCL22 was further investigated using immunohistochemistry. Staining for CCL22 was noted to be strongest within the intima and adventitia of biopsies from the body of AAAs (Figure 2, A–L, and Figure 3, A–G). There was much less staining found in biopsies from the neck of AAAs and minimal staining was demonstrated in atheroma samples (Figure 2). Sparse staining for the CCL22 receptor (CCR4) was noted in all three tissue types (Figure 2). CCL22 and CCR4 staining was demonstrated in areas also staining for a macrophage and a neutrophil marker (Figure 3). Neutrophil staining was particularly prominent in the section examined (Figure 3).
Figure 2.
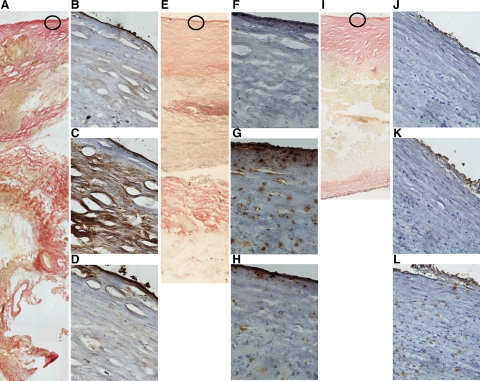
Examples of immunohistochemical staining for CCL22 and its receptor, CCR4, in AAA (body, A, B, C, and D, and neck, E, F, G, and H), and atheroma (I, J, K, and L) biopsies. Shown are examples of sections stained by the Verhoeff-van Gieson method at ×10 magnification (A, E, and I). IHC for the regions highlighted in circles at ×40 magnification are also shown for CCL22 (C, G, and K) and CCR4 (D, H, and L). Sections stained with goat IgG control Abs (B, F, and J) were also included. Staining for CCL22 is most marked in the intima of biopsies from the body of an AAA (C), sparse in the intima of an AAA neck biopsy (G) and minimal in the intima of an atheroma biopsy (K). Sparse staining for the CCL22 receptor, CCR4, was found in biopsies from all three tissue types (D, H, and L).
Figure 3.
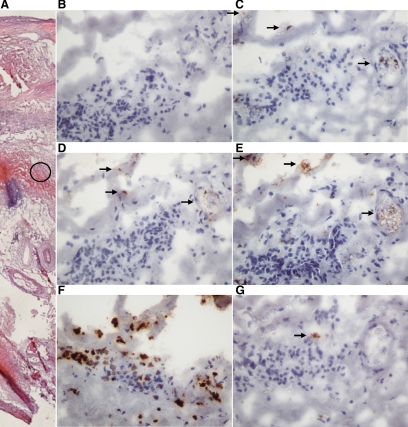
Examples of high power (×40) photomicrographs of staining for CCL22 (D), CCR4 (E), macrophages (C), neutrophils (F), and mast cells (G) in a biopsy from the body of an AAA. Also shown is an H&E-stained section of the biopsy to illustrate the region the high power images are taken from highlighted with a black circle (A). B: Negative control using an isotype control Ab. Arrows indicate stained areas.
Serum CCL22 Was Associated with AAA
Given the consistently high concentrations of CCL22 in AAA biopsies we next assessed whether circulating concentrations of this cytokine were associated with aortic dilatation in study 4. We measured the serum concentrations of CCL22 in 315 men with AAA and 713 controls without aortic dilatation. The risk factors and circulating cytokine concentrations for these men are shown in Table 3. Serum concentrations of CCL22 were higher in patients with AAAs, as compared with controls (Table 3). Serum CCL22 concentrations were independently associated with AAA after adjusting for other risk factors (odds ratio 1.51, 95% confidence intervals 1.21–1.88, per 0.4 ng/ml, Table 4). Patients with CCL22 concentrations in the upper tertile had an adjusted odds ratio for AAA of 2.71 (95% confidence interval 1.86–3.95) and 2.07 (95% confidence intervals 1.46–2.94) compared with patients with CCL22 concentrations in the lower and middle tertiles, respectively. Area under the curve for receiver operator characteristic curves (95% confidence intervals) in predicting the presence of AAA were 0.64 (0.60−0.68) and 0.59 (0.56−0.63) for serum CCL22 and C-reactive protein, respectively. Serum CCL22 was also higher in patients with large AAAs (≥40 mm) compared with controls (Table 3) and independently associated with AAA when a definition of ≥40 mm was used (Table 4). The correlation between serum CCL22 and aortic diameter was however weak, r = 0.19, P < 0.01. The plasma concentration of IL6, which was not associated with AAA in either of the cytokine array studies (see Supplemental Table S1 at http://ajp.amjpathol.org), was also measured. No association between circulating IL6 and AAA was found (Table 3), which was in keeping with the cytokine array results from studies 1 and 2.
Table 3.
Comparison of Patients with and without AAA Undergoing Circulating Cytokine Measurements
| Characteristic | <30 mm | 30–39 mm | ≥40 mm | P value |
|---|---|---|---|---|
| Number | 713 | 245 | 70 | |
| Aortic diameter (mm) | 21.9 (20.2–23.1) | 32.5 (31.0–34.6) | 45.0 (42.1–49.4) | <0.01 |
| Age (years) | 70.6 (68.0–74.2) | 72.2 (68.9–75.2) | 71.1 (68.6–74.7) | <0.01 |
| Hypertension | 276 (39%) | 124 (51%) | 37 (53%) | <0.01 |
| Diabetes mellitus | 49 (7%) | 22 (9%) | 9 (13%) | 0.15 |
| Dyslipidaemia | 263 (37%) | 127 (52%) | 28 (40%) | <0.01 |
| Ever smoker | 449 (63%) | 206 (84%) | 61 (87%) | <0.01 |
| CHD | 123 (17%) | 87 (36%) | 30 (43%) | <0.01 |
| WHR | 0.95 (0.91−0.99) | 0.96 (0.93–1.01) | 0.98 (0.93–1.05) | <0.01 |
| CCL22 (ng/ml) | 0.56 (0.45−0.70) | 0.67 (0.52−0.82) | 0.74 (0.56−0.89) | <0.01 |
| IL-6 (pg/ml) | 14.2 (5.2–38.8) | 9.3 (5.2–31.2) | 17.3 (4.6–38.7) | 0.34 |
| CRP (mg/L) | 1.77 (0.95–3.57) | 2.54 (1.24–5.11) | 2.75 (1.43–5.56) | <0.01 |
| Creatinine (μM) | 89 (78–100) | 92 (80–111) | 103 (90–134) | <0.01 |
Data are shown comparing subjects without AAAs (<30 mm), with small AAAs (30 to 40 mm) and larger AAAs (≥40 mm). Nominal variables are presented as numbers (%) and compared by chi-square. Continuous variables are presented as median (inter-quartile range) and compared by Kruskal Wallis test. CHD = Coronary heart disease; WHR = Waist to hip ratio.
Table 4.
Independent Association of Clinical and Circulating Factors with AAA in 1028 Men
| Characteristic | AAA ≥ 30 mm
|
AAA ≥ 40 mm
|
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Age per 4 years* | 1.20 | 1.04–1.38 | 0.01 | 1.01 | 0.79–1.30 | 0.92 |
| Hypertension | 1.21 | 0.88–1.65 | 0.24 | 1.21 | 0.70–2.11 | 0.49 |
| Diabetes mellitus | 1.08 | 0.64–1.82 | 0.78 | 1.32 | 0.59–2.95 | 0.51 |
| Dyslipidaemia | 1.22 | 0.89–1.68 | 0.22 | 0.58 | 0.33–1.04 | 0.07 |
| CHD | 2.23 | 1.53–3.25 | <0.01 | 2.92 | 1.57–5.45 | <0.01 |
| Ever smoker | 2.87 | 1.98–4.15 | <0.01 | 2.72 | 1.28–5.78 | <0.09 |
| Aspirin prescription | 1.14 | 0.81–1.61 | 0.46 | 0.92 | 0.50–1.70 | 0.80 |
| Waist-hip ratio per 0.06* | 1.39 | 1.20–1.61 | <0.01 | 1.63 | 1.28–2.07 | <0.01 |
| Creatinine per 40 μmol/L* | 1.10 | 0.97–1.25 | 0.13 | 1.18 | 1.02–1.36 | 0.02 |
| CRP per 7 mg/L* | 1.15 | 0.98–1.34 | 0.08 | 0.86 | 0.60–1.23 | 0.41 |
| CCL22 per 0.4 ng/ml* | 1.51 | 1.21–1.88 | <0.01 | 1.33 | 1.08–1.62 | <0.03 |
AAA = Abdominal aortic aneurysm. CHD = Coronary heart disease. CCL22 = chemokine CC motif ligand 22. CRP = C-reactive protein. For nominal variables the comparisons are to subjects without the risk factor.
Approximately one standard deviation.
Discussion
Cytokines have been linked with AAA in numerous previous studies, but in the current investigation we have examined a large number of cytokines in carefully controlled biopsies to assess those cytokines reproducibly associated with aortic dilatation. In an initial screening study we identified 3 of 79 cytokines investigated to be consistently expressed at higher concentrations within AAA compared with two types of control biopsies. Only one of these cytokines, CCL22, had not previously been linked with AAA. We therefore validated the expression of this cytokine in additional biopsies and subsequently demonstrated increased concentrations within the circulation of men with AAA. Serum concentrations of CCL22 were independently associated with AAA after adjusting for other risk factors including the general inflammatory marker C-reactive protein, and other potential confounding factors (age, hypertension, coronary heart disease, dyslipidemia, smoking, diabetes, aspirin prescription, serum creatinine, waist-hip ratio). Thus our study suggests the consistent association of tissue and circulating concentrations of a novel cytokine with AAA.
Previous studies examining expression of cytokines in human AAA biopsies have principally been limited to a small number of candidates of interest, including CCL2,17,19,20 IL1B,17,19,21,22,23,24,25 IL6,17,19,23,25,26,27 IL8,17,19,20,25,28 IL10,17,19,23,25 IL12,17,23 INFγ,17,23,28 prostaglandin E225,27,29 and TNFα.17,19,21,22,23,24,26,30 One study reported the use of an antibody array to assess the relative expression of 42 cytokines.19 These studies illustrate the large number of challenges in carrying out this type of investigation. Many investigators used postmortem aorta biopsies removed from patients dying from non-cardiovascular causes18,19,20,21,22,24,25,29,30 as controls, with obvious concerns that the samples may not be representative of the in vivo situation. Some investigators have also included or preferred biopsies of atherothrombosis as controls17,20,21,23,24,26,27,28,30 In most cases the control patients are not matched to those with AAA17,18,19,24,26,30 or this information is not presented.20,21,22,25,27,28,29 Matching of cases and controls for such studies is difficult to achieve for a number of reasons. Firstly organ donors, subjects undergoing postmortems and those presenting with lower limb ischemia are expected to have differences in age at presentation and other risk factors from those presenting with AAA. Secondly aortic biopsies from any sources are increasingly difficult to obtain at any single center due to reduction in open aortic surgery. In the few studies reporting matching of age and gender it would be expected that there would be other differences between cases and controls such as medication prescription.23 In an attempt to minimize the concerns regarding controls careful matching of cases and controls was used in study 1, including matching of age, other risk factors and medications as far as was possible. Furthermore, in study 2 within-patient controls were used. This approach allowed more appropriate identification of potential candidate cytokines.
Histological examination illustrated that while biopsies from the neck of the AAA do not have completely normal morphology they show relative preservation of intimal, medial, and adventitial architecture, which is markedly disparate to biopsies taken from the body of the AAA. AAA neck biopsies thus act as a critical internal control since they are matched in everyway to the body samples except for the macroscopic presence of aortic dilatation and the associated histological changes of medial destruction and inflammation. Such complete matching is not possible with either normal aortic biopsies (eg, from organ donors or postmortem) or those from patients with atherothrombosis.
There is current controversy regarding whether T cell subtypes such as Th-1 or Th-2 are important in AAA pathogenesis.3,31,32,33 Some of the findings from this study are suggestive of a Th-1 response being present in AAA biopsies. We confirmed the up-regulation of IL1B17,19,21,22,24 and IL817,18,19,20 demonstrated in some previous studies, both of these cytokines being associated with a Th-1 response.17,34 We identified higher concentrations of the Th-1 associated cytokine INFγ in AAA biopsies compared with those from the nondilated proximal aorta, however, relative expression of INFγ was reduced in AAA biopsies compared with those from atherothrombosis. Two of three previous studies reported upregulation of INFγ compared with those of atherothrombosis.17,23,28 The one study to report no relative increase of INFγ in biopsies of AAA was the only study to match patients and controls for age and gender.23
This study is the first to identify an association of both tissue and circulating concentrations of CCL22 with AAA. One previous study reported similar concentrations of CCL22 in biopsies from AAA and cadaveric kidney donors but no previous study has reported assessment of circulating concentrations of CCL22 in patients with AAA.19 CCL22 was originally identified in macrophages (and called macrophage-derived chemokine), but subsequently lymphocytes and dendritic cells have also been demonstrated to produce this chemokine.35,36 CCL22 has chemoattractant effects on a variety of inflammatory cells, including macrophages, T cells, and natural killer cells.36 Of particular interest to the present discussion CCL22 has been implicated in generation of a Th-2 immune response. CCL22 preferentially attracts Th-2 CD4+ cells and its production from such cells is stimulated by recognized Th-2 cytokines, such as IL4.37 Production of CCL22 is inhibited by Th-1 cytokines such as INFγ.36 High circulating concentrations of CCL22 have also been reported within the circulation of patients with mycosis fungoides and atopic dermatitis, which are both diseases characterized by Th-2 focused immune responses.38 In contrast, multiple sclerosis and Crohn’s disease, disorders characterized by Th-1 responses, have been reported to have low concentrations of circulating CCL22.38 Immunohistochemistry studies demonstrated CCL22 staining to be adjacent to that for macrophages, which also stained positively for the CCL22 receptor CCR4. These findings suggest that CCL22 might be stimulating macrophage infiltration within AAAs. We also demonstrated strong staining for neutrophils within the area of CCL22/CCR4 staining. Thus overall our findings do not support a Th-1- or Th-2-specific response in AAA and suggest a variety of different cytokines and immune pathways are most likely involved.
This study has a number of strengths and weaknesses, some of which are outlined above. The small sample size used to screen biopsies for cytokine of interest is typical of studies of this type.19,21,23,28,29 It would however have been ideal to have a larger cohort, particularly given the large number of cytokines assessed and the risk of false positive discoveries. Our choice of patients to include was however limited by our strict entry criteria designed to achieve appropriate matching of groups. We did design our study to reduce the potential for false discovery by assessing relative cytokine concentrations with respect to both biopsies of atherothrombosis and macroscopically normal aorta and selecting only those up-regulated consistently, after correcting for multiple testing. The novel cytokine identified by the protein arrays used in this study was validated on other samples using a further assessment technique, ie, ELISA. The control samples included in this validation study were endarterectomy samples and therefore not completely comparable with full thickness aortic biopsies. Aortic atherosclerotic samples from matched patients are difficult to obtain due to the reduction in open aortic surgery. CCL22 was however shown to be consistently up-regulated with the serum of a cohort of men larger than any previous study investigating the association of circulating cytokines with AAA.6,7,8,39
In conclusion this study illustrates a relatively new approach to identifying appropriate cytokines of pathogenic relevance to AAA. This study demonstrates the novel association of CCL22 with AAA. Mechanistic studies using animal models of aortic aneurysm may shed further light on the importance of this cytokine. It should be noted though that previous animal studies of other cytokines, such as INFγ, have demonstrated diametrically opposite findings in different models, illustrating the difficulty in relating rodent studies to human pathology.32,33 It is hoped in the future that using similar approaches to those described in this study, including a variety of new proteomic techniques, it may be possible to identify a group of circulating biomarkers with specific association with AAA of value in the diagnosis, prognosis, or predicting treatment options in AAA.
Footnotes
Address reprint requests to Professor Jonathan Golledge, MChir, Director, The Vascular Biology Unit, Department of Surgery, School of Medicine, James Cook University Townsville, QLD, Australia 4811. E-mail: jonathan.golledge@jcu.edu.au.
Supported by the NIH (RO1 HL080010), Smart State National and International Research Alliances Program from the Queensland Government and James Cook University. J.G. and P.N. hold Practitioner Fellowships from the National Health and Medical Research Council, Australia (431503 and 45805).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Kent KC, Zwolak RM, Jaff MR, Hollenbeck ST, Thompson RW, Schermerhorn ML, Sicard GA, Riles TS, Cronenwett JL, Society for Vascular Surgery, American Association of Vascular Surgery, Society for Vascular Medicine and Biology Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39:267–269. doi: 10.1016/j.jvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–2613. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med. 2000;38:1161–1164. doi: 10.1515/CCLM.2000.178. [DOI] [PubMed] [Google Scholar]
- Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjälä H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- Dawson J, Cockerill GW, Choke E, Belli AM, Loftus I, Thompson MM. Aortic aneurysms secrete interleukin-6 into the circulation. J Vasc Surg. 2007;45:350–356. doi: 10.1016/j.jvs.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Feng L, Matsumoto C, Schwartz A, Schmidt AM, Stern DM, Pile-Spellman J. Chronic vascular inflammation in patients with type 2 diabetes: endothelial biopsy and RT-PCR analysis. Diabetes Care. 2005;28:379–384. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- Neri Serneri GG, Boddi M, Modesti PA, Coppo M, Cecioni I, Toscano T, Papa ML, Bandinelli M, Lisi GF, Chiavarelli M. Cardiac angiotensin II participates in coronary microvessel inflammation of unstable angina and strengthens the immunomediated component. Circ Res. 2004;94:1630–1637. doi: 10.1161/01.RES.0000130944.49657.b8. [DOI] [PubMed] [Google Scholar]
- Rehring TF, Stolcpart RS, Hollis HW, Jr, Society for Vascular Surgery Pharmacologic risk factor management in peripheral arterial disease: a vade mecum for vascular surgeons. J Vasc Surg. 2008;47:1108–1115. doi: 10.1016/j.jvs.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Norman PE, Flicker L, Almeida OP, Hankey GJ, Hyde Z, Jamrozik K. Cohort Profile: the Health In Men Study (HIMS). Int J Epidemiol. 2009;38:48–52. doi: 10.1093/ije/dyn041. [DOI] [PubMed] [Google Scholar]
- Golledge J, Jayalath R, Oliver L, Parr A, Schurgers L, Clancy P. Relationship between CT anthropometric measurements, adipokines and abdominal aortic calcification. Atherosclerosis. 2008;197:428–434. doi: 10.1016/j.atherosclerosis.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran CS, McCann M, Karan M, Norman P, Ketheesan N, Golledge J. Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation. 2005;111:3119–3125. doi: 10.1161/CIRCULATIONAHA.104.464727. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–57. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc B, 1995;57:289–300. [Google Scholar]
- Lindeman JH, Abdul-Hussien H, Schaapherder AF, Van Bockel JH, Von der Thüsen JH, Roelen DL, Kleemann R. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond) 2008;114:687–697. doi: 10.1042/CS20070352. [DOI] [PubMed] [Google Scholar]
- Treska V, Kocova J, Boudova L, Neprasova P, Topolcan O, Pecen L, Tonar Z. Inflammation in the wall of the abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cyto Cell Mol Ther. 2002;7:91–97. doi: 10.1080/13684730310001652. [DOI] [PubMed] [Google Scholar]
- Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45:574–580. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Pearce WH, Shah MR, Parikh D, Evanoff HL, Haines GK, Burdick MD, Strieter RM. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993;142:1423–1431. [PMC free article] [PubMed] [Google Scholar]
- Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:II224–II227. [PubMed] [Google Scholar]
- Hamano K, Li TS, Takahashi M, Kobayashi T, Shirasawa B, Ito H, Zempo N. Enhanced tumor necrosis factor- alpha expression in small sized abdominal aortic aneurysms. World J Surg. 2003;27:476–480. doi: 10.1007/s00268-002-6690-0. [DOI] [PubMed] [Google Scholar]
- Davis VA, Persidskaia RN, Baca-Regen LM, Fiotti N, Halloran BG, Baxter BT. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–156. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- Pearce WH, Sweis I, Yao JS, McCarthy WJ, Koch AE. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg. 1992;16:784–789. [PubMed] [Google Scholar]
- Cheuk BL, Cheng SW. Differential secretion of prostaglandin E(2), thromboxane A(2) and interleukin-6 in intact and ruptured abdominal aortic aneurysms. Int J Mol Med. 2007;20:391–395. [PubMed] [Google Scholar]
- Shteinberg D, Halak M, Shapiro S, Kinarty A, Sobol E, Lahat N, Karmeli R. Abdominal aortic aneurysm and aortic occlusive disease: a comparison of risk factors and inflammatory response. Eur J Vasc Endovasc Surg. 2000;20:462–465. doi: 10.1053/ejvs.2000.1210. [DOI] [PubMed] [Google Scholar]
- Reilly JM, Miralles M, Wester WN, Sicard GA. Differential expression of prostaglandin E2 and interleukin-6 in occlusive and aneurysmal aortic disease. Surgery. 1999;126:624–627. [PubMed] [Google Scholar]
- Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159–162. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- Holmes DR, Wester W, Thompson RW, Reilly JM. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J Vasc Surg. 1997;25:810–815. doi: 10.1016/s0741-5214(97)70210-6. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nakamura M, Satoh M, Nakajima T, Izumoto H, Maesawa C, Kawazoe K, Masuda T, Hiramori K. Expression and localization of tumour necrosis factor-alpha and its converting enzyme in human abdominal aortic aneurysm. Clin Sci (Lond) 2004;106:301–306. doi: 10.1042/CS20030189. [DOI] [PubMed] [Google Scholar]
- Galle C, Schandené L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin Exp Immunol. 2005;142:519–527. doi: 10.1111/j.1365-2249.2005.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-γ signaling induce aneurysms in allografted aortas. J Clin Invest. 2004;114:300–308. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- Hata H, Yoshimoto T, Hayashi N, Hada T, Nakanishi K. IL-18 together with anti-CD3 antibody induces human Th1 cells to produce Th1- and Th2-cytokines and IL-8. Int Immunol. 2004;16:1733–1739. doi: 10.1093/intimm/dxh174. [DOI] [PubMed] [Google Scholar]
- Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC). J Leukoc Biol. 2000;68:400–404. [PubMed] [Google Scholar]
- Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG., 3rd STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- Galli G, Chantry D, Annunziato F, Romagnani P, Cosmi L, Lazzeri E, Manetti R, Maggi E, Gray PW, Romagnani S. Macrophage-derived chemokine production by activated human T cells in vitro and in vivo: preferential association with the production of type 2 cytokines. Eur J Immunol. 2000;30:204–210. doi: 10.1002/1521-4141(200001)30:1<204::AID-IMMU204>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118:2382–2392. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]