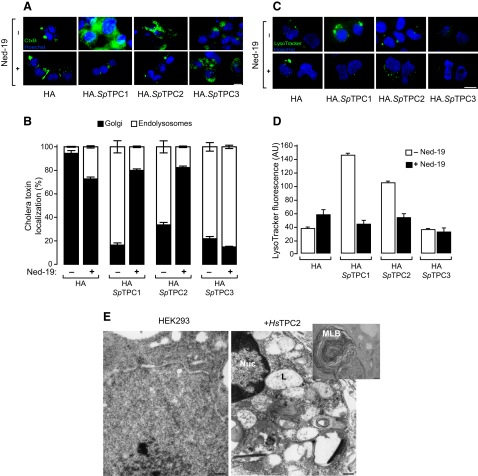
Figure 4.
Two-Pore Channel Overexpression Causes Changes in Endolysosomal Trafficking and Morphology
(A) Lipid endocytosis and recycling in HA.SpTPC-overexpressing HEK293 cells assessed by Alexa Fluor 568-cholera toxin B subunit (CtxB; green). Nuclei are labeled with Hoechst 33342 (Hoechst, blue). Scale bar corresponds to 5 μm. Endolysosomal localization was confirmed by colocalization with endocytosed high molecular weight rhodamine dextran (data not shown); Golgi localization by a single perinuclear distribution is not overlapping with the dextran.
(B) Summary of results from experiments in (A) corresponding to three separate experiments with a minimum of 50 cells analyzed per experiment.
(C) Pattern of lysosomal staining by LysoTracker Green (LysoTracker, green) in HA.SpTPC-overexpressing HEK293 cells, in the absence or presence of NAADP receptor antagonist Ned-19 (10 μM, 12 hr). Nuclei were labeled with Hoechst 33342 (Hoechst, blue). Scale bars correspond to 5 μm.
(D) Summary of results from experiments in (C) corresponding to three separate experiments, with a minimum of 50 cells analyzed per experiment. To allow meaningful comparison between images, we standardized LysoTracker loading conditions and imaging protocols for all cell types. pH differences between cell types were negligible, as assessed by fluorescein-dextran fluorescence (data not shown).
(E) Lysosomal storage disease phenotype in HA.HsTPC2-overexpressing HEK293 cells visualized by electron microscopy. Inset is a magnification of one region of a HA.HsTPC2-overexpressing cell. Scale bars correspond to 200 nm. The following abbreviations are used: Nuc, nucleus; MLB, multiple lamellar inclusion body; L, lysosome.
Data are represented as mean ± SEM. See also Figure S4.