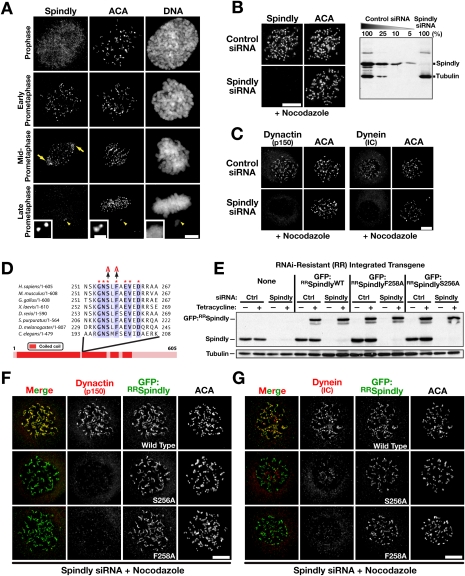
Figure 1.
Single amino acid changes in Spindly uncouple its kinetochore localization from dynein/dynactin recruitment. (A) Mitotic HeLa cells fixed and immunostained for Spindly and centromere antigens (ACA). In addition to kinetochores, Spindly is also visible at spindle poles (arrows) during chromosome alignment. Spindly is absent from kinetochores at the metaphase plate, but is detectable at an unaligned kinetochore pair (arrowhead). (B) Immunoblot and immunofluorescence 48 h after transfection of HeLa cells with control and Spindly siRNA. α-Tubulin was used as a loading control for the immunoblot. Cells were treated with nocodazole for 4 h prior to fixation and immunostaining. (C) Localization of dynein intermediate chains and the dynactin subunit p150Glued at unattached kinetochores in control and Spindly siRNA-treated cells. Cells were incubated in nocodazole for 4 h to accumulate dynein/dynactin at kinetochores (see also Supplemental Fig. S1D). (D) Sequence alignment of the highly conserved motif in the Spindly protein family. The conserved serine and phenylalanine (S256 and F258 in human Spindly) that were individually mutated to alanine are indicated. (E) Immunoblot monitoring endogenous and RNAi-resistant (RR) transgenic Spindly expression. Cells were treated with control or Spindly siRNA for 22 h followed by induction with 0.2 μg/mL tetracycline for 8 h. α-Tubulin is used as a loading control. (F,G) Cell lines expressing GFP:RRSpindlyWT or the point mutants depicted in D immunostained for GFP, centromere antigens (ACA), and either the dynactin subunit p150Glued (F) or dynein intermediate chains using the monoclonal antibody 70.1 (G). Cells were treated with Spindly siRNA for 24 h, transgene expression was induced with tetracycline for 16 h, and nocodazole was added for 4 h prior to fixation. Bars, 5 μm; inset in A, 1 μm.