Table 1.
Carbonyl propargylation from the alcohol oxidation level by ruthenium-catalyzed transfer hydrogenation.a
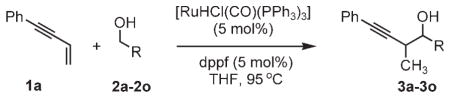 | ||
| 2a, R =p-NO2Ph | 2f, R =p-BrPh | 2k, R =geranyl |
| 2b, R =phenyl | 2g, R =2-furyl | 2l, R =crotyl |
| 2c, R =p-MeOPh | 2h, R =3-indolyl | 2m, R =cyclopropyl |
| 2d, R =o-MeOPh | 2i, R =2-(6-BrPy) | 2n, R =benzyl |
| 2e, R =5-piperonyl | 2j, R =cinnamyl | 2o, R =n-pentyl |
| Coupling to benzylic alcohols | ||
 |
 |
 |
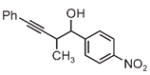
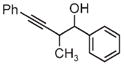
| 3a | 3b | 3c |
| 65% yield | 81% yield | 81% yield |
| 1:1 d.r. | 1:1 d.r. | 1:1 d.r. |
 |
 |
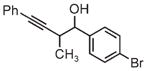 |
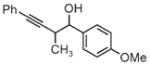
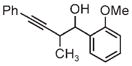
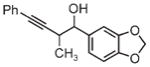
| 3d | 3e | 3f |
| 91% yield | 83% yield | 73% yield |
| 2:1 d.r. | 2:1 d.r. | 1:1 d.r. |
 |
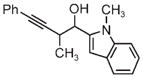 |
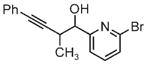 |
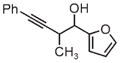
| 3g | 3h | 3i |
| 71% yield | 94% yield | 42% yield |
| 1.5:1 d.r. | 1:1 d.r. | 1.3:1 d.r. |
| Coupling to allylic alcohols | ||
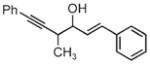 |
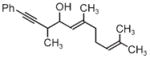 |
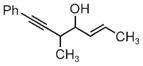 |
| 3j | 3k | 3l |
| 68% yield | 63% yield | 72% yield |
| 1:1 d.r. | 1.5:1 d.r. | 2:1 d.r. |
| Coupling to aliphatic alcohols | ||
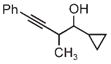 |
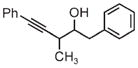 |
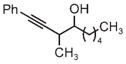 |
| 3m | 3n | 3o |
| 75% yield | 70% yield | 72% yield |
| 2:1 d.r. | 1:1 d.r. | 2:1 d.r. |
Yields of isolated material. Standard reaction conditions employed 1 equivalent of alcohol/aldehyde and 2 equivalents of enyne. See the Supporting Information for detailed experimental procedures. Py =pyridine.
