Table 2.
Coupling of enynes 1b–1g to benzyl alcohol 2b by ruthenium-catalyzed transfer hydrogenation.a
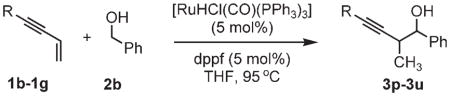 | ||
| 1b, R =2-thienyl | 1d, R =TBSO(CH2)4 | 1f, R =TBSOC(CH3)2 |
| 1c, R =BocNH(CH2)2 | 1e, R =TBSOCH2 | 1g, R =cyclohexyl |
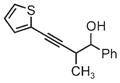 |
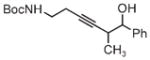 |
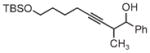 |
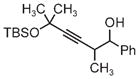
| 3pb | 3q | 3r |
| 71% yield | 54% yield | 63% yield |
| 1:1 d.r. | 1:1 d.r. | 1:1 d.r. |
 |
 |
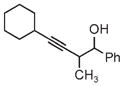 |
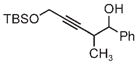
| 3s | 3tb | 3ub |
| 78% yield | 56% yield | 70% yield |
| 1.5:1 d.r. | 1:1 d.r. | 1:1 d.r. |
See the footnotes of Table 1 for details.
m-NO2BzOH (5 mol%) was employed as a cocatalyst. Boc =tert-butyloxycarbonyl, Bz =benzyl, TBS =tert-butyldimethylsilyl.
