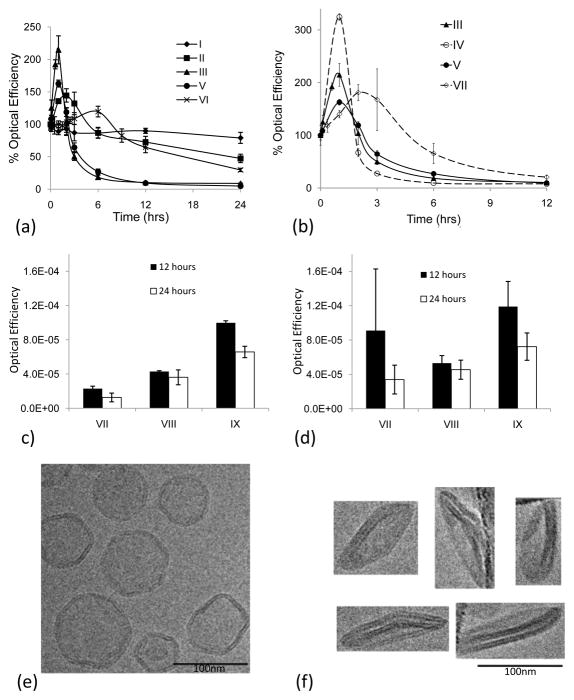
Fig. 5.
5a–d. Optical image efficiency of superficial tissue following injection of AF-750 loaded liposomes, normalized by initial fluorescence for each formulation. 5a) Optical efficiency of superficial tissue (as an indicator of blood concentration and stability) after injection of isotonic liposomes containing negatively-charged lipid-PEG moieties. Longest circulation for high cholesterol and HSPC (I and II); shortest for lysolipid and DPPC (III, IV). Two temperature sensitive formulations (II and VI) demonstrate extended circulation. 5b) Optical efficiency of superficial tissue (as an indicator of blood concentration and stability) after injection of temperature-sensitive formulations containing uncharged lipopolymer (Ceramide-PEG2k) and negatively-charged lipopolymer (DSPE-PEG2k) (III vs IV and V vs VII). 5c) Optical efficiency of superficial tissue (as an indicator of blood concentration and stability) after injection of iso-osmolar (300 mOsm (VII)) and hypo-osmolar (38 mOsm (VIII) and 10 mOsm (IX) liposomes). Decreased osmolarity increases duration of fluorescence. 5d) Optical efficiency of bladder (as an indicator of particle stability). Decreasing the osmolarity of the entrapped AF-750 solution increases fluorophore retention, as shown by prolonged fluorophore clearance to the bladder. 5e–5f. TEM imaging of DPPC:DSPC:Ceramide-PEG2k (85.5/9.5/5 molar ratio) liposomes at room temperature in 300 mOsm PBS. 5e) Isotonic liposomes prepared in 300 mOsm PBS; angular spherical vesicles are produced due to the high gel-to-liquid-crystalline phase transition temperatures of the liposome membranes (>42°C). 5f) Hypotonic liposomes prepared in 10 mOsm PBS yield flat, circular disk-like vesicles.