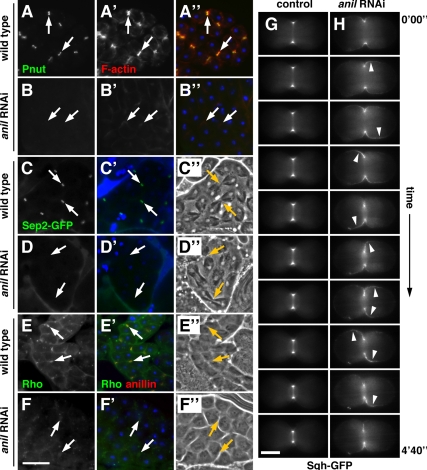
Figure 4.
Anillin is required for recruitment of septins and stabilization of Rho and myosin II at the cleavage furrow. (A–A″ and B–B″) Dividing spermatocytes stained for the septin peanut (Pnut; green), F-actin (red), and DNA (blue). In cleavage furrows (arrows) of wild-type spermatocytes, peanut (A) and F-actin (A′) colocalize in cleavage furrows (A″), whereas in dsRNA-expressing cells peanut is absent (B) and F-actin appears diffuse (B′). (C–D′) Dividing spermatocytes expressing Sep2-GFP (green) and stained for DNA (blue) with corresponding phase-contrast micrographs (C″ and D″). Sep2-GFP localizes to cleavage furrows in wild-type cells (C), but is absent in dsRNA-expressing cells (D). (E–F′) Dividing spermatocytes stained for Rho (green), anillin (red), and DNA (blue) with corresponding phase contrast micrographs (E″ and F″). In cleavage furrows of wild-type spermatocytes, Rho (E) and anillin colocalize in cleavage furrows (E′), whereas in dsRNA-expressing cells Rho appears diffuse (F) and anillin is absent (F′). Bar, 20 μm. (G and H) Time-lapse images showing the change in localization of Sqh-GFP (myosin regulatory light chain) during cytokinesis. In control cells, Sqh-GFP is tightly associated with the furrow (G), whereas in dsRNA-expressing cells Sqh-GFP moves around the cell cortex in an oscillatory manner (H). The images shown were taken at 40-s intervals. Corresponding full-length videos are available online (Supplemental Videos 1 and 2). Bar, 5 μm.