Abstract
A microsomal adenosine triphosphatase (ATPase) that requires both sodium and potassium ions is thought to be identical with, or an integral part of, the active cation transport system located in cell membranes. Attempts to isolate and purify (Na+ + K+)-ATPase have met with limited success because solubilization of microsomal protein causes partial, if not complete, loss of enzymatic activity. We now report the isolation from rat kidney microsomes of proteins which, though enzymatically inactive, could still be identified as components of the (Na+ + K+)-ATPase system.
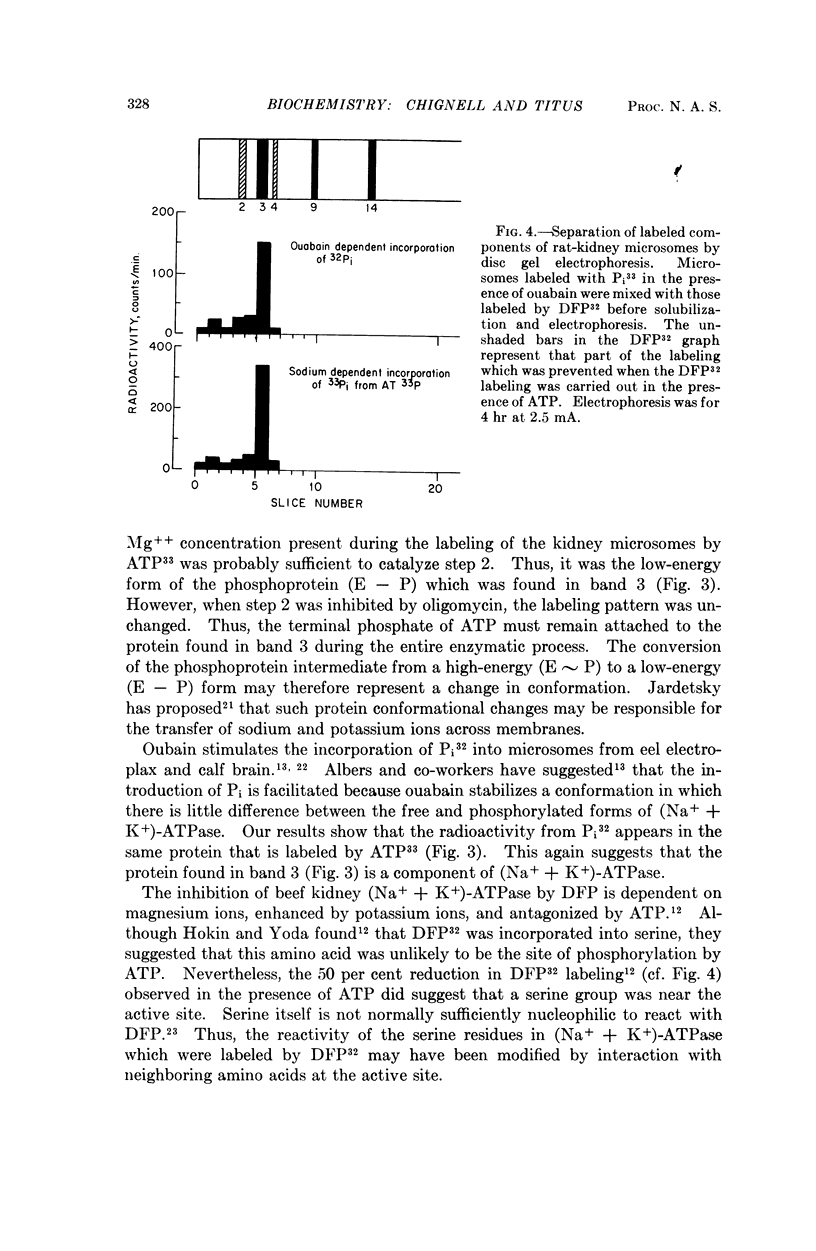
Phosphoproteins known to be intermediates in the hydrolysis of ATP by (Na+ + K+)-ATPase were prepared by incubating rat kidney microsomes with γ-labeled ATP33 in the presence of sodium or with P32-orthophosphate in the presence of ouabain. After the P32- and P33-labeled microsomes had been dissolved in phenol-acetic acid-urea, the resultant solutions were mixed and subjected to polyacrylamide gel electrophoresis. The radioactivity from both phosphorus isotopes was found almost exclusively in one of the resultant 21 protein bands. In contrast, the radioactive protein from DFP32-labeled microsomes moved slightly faster than the radioactive protein from microsomes labeled with P33-orthophosphate in the presence of ouabain. DFP inhibits (Na+ + K+)-ATPase by reacting with a nucleophilic site at or near the active site. These results suggest that while a single protein component of (Na+ + K+)-ATPase accepts the terminal phosphate from ATP, the final splitting of this phosphoprotein intermediate may be catalyzed by nucleophilic sites on a second protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers R. W., Koval G. J., Siegel Studies on the interaction of ouabain and other cardio-active steroids with sodium-potassium-activated adenosine triphosphatase. Mol Pharmacol. 1968 Jul;4(4):324–336. [PubMed] [Google Scholar]
- Chignell C. F., Titus E. The effect of hydroxylamine and N-methylhydroxylamine on beef brain microsomal (Na+ + K+)-ATPase. Biochim Biophys Acta. 1968 Jun 4;159(2):345–351. doi: 10.1016/0005-2744(68)90083-1. [DOI] [PubMed] [Google Scholar]
- Chrambach A. Device for sectioning of polyacrylamide gel cylinders and its use in determining biological activity in the sections. Anal Biochem. 1966 Jun;15(3):544–548. doi: 10.1016/0003-2697(66)90121-7. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Mahler H. R. Resolution of insoluble proteins in rat brain subcellular fractions. Arch Biochem Biophys. 1967 May;120(2):384–396. doi: 10.1016/0003-9861(67)90255-x. [DOI] [PubMed] [Google Scholar]
- Fahn S., Hurley M. R., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. II. Effects of N-ethylmaleimide and other sulfhydryl reagents. J Biol Chem. 1966 Apr 25;241(8):1890–1895. [PubMed] [Google Scholar]
- Fahn S., Koval G. J., Albers R. W. Sodium-potassium-activated adenosine triphosphatase of Electrophorus electric organ. I. An associated sodium-activated transphosphorylation. J Biol Chem. 1966 Apr 25;241(8):1882–1889. [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- GIBBS R., RODDY P. M., TITUS E. PREPARATION, ASSAY, AND PROPERTIES OF AN NA+- AND K+-REQUIRING ADENOSINE TRIPHOSPHATASE FROM BEEF BRAIN. J Biol Chem. 1965 May;240:2181–2187. [PubMed] [Google Scholar]
- HOKIN L. E., YODA A. INHIBITION BY DIISOPROPYLFLUOROPHOSPHATE OF A KIDNEY TRANSPORT ADENOSINE TRIPHOSPHATASE BY PHOSPHORYLATION OF A SERINE RESIDUE. Proc Natl Acad Sci U S A. 1964 Aug;52:454–461. doi: 10.1073/pnas.52.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi C. E., Titus E. Kinetics of oligomycin inhibition of sodium- and potassium-activated adenosine triphosphatase from beef brain. Mol Pharmacol. 1968 Nov;4(6):591–599. [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966 Aug 27;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Lindenmayer G. E., Laughter A. H., Schwartz A. Incorporation of inorganic phosphate-32 into a Na+, K+-ATPase preparation: stimulation by ouabain. Arch Biochem Biophys. 1968 Sep 20;127(1):187–192. doi: 10.1016/0003-9861(68)90215-4. [DOI] [PubMed] [Google Scholar]
- Nakao T., Tashima Y., Nagano K., Nakao M. Highly specific sodium-potassium-activated adenosine triphosphatase from various tissues of rabbit. Biochem Biophys Res Commun. 1965 Jun 9;19(6):755–758. doi: 10.1016/0006-291x(65)90323-2. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Preparation from mammallian brain and kidney of the enzyme system involved in active transport of Na ions and K ions. Biochim Biophys Acta. 1962 Apr 9;58:314–325. doi: 10.1016/0006-3002(62)91015-6. [DOI] [PubMed] [Google Scholar]
- Schoner W., von Ilberg C., Kramer R., Seubert W. On the mechanism of Na+- and K+-stimulated hydrolysis of adenosine triphosphate. 1. Purification and properties of a Na+-and K+-activated ATPase from ox brain. Eur J Biochem. 1967 May;1(3):334–343. doi: 10.1007/978-3-662-25813-2_45. [DOI] [PubMed] [Google Scholar]
- Stahl W. L. Sodium stimulated [14C]adenosine diphosphate-adenosine triphosphate exchange activity in brain microsomes. J Neurochem. 1968 Jun;15(6):511–518. doi: 10.1111/j.1471-4159.1968.tb08948.x. [DOI] [PubMed] [Google Scholar]