Abstract
Background
Lycopene, selenium, and vitamin E are three micronutrients commonly consumed and supplemented by men diagnosed with prostate cancer. However, it is not clear whether consumption of these compounds, alone or in combination, results in improved outcomes.
Methodology/Principal Findings
We evaluated the effects of dietary lycopene (250 mg/kg diet), selenium (methylselenocysteine, 1 mg/kg diet), and vitamin E (γ-tocopherol, 200 mg/kg diet) alone and in combination on the growth of androgen-dependent Dunning R3327-H rat prostate adenocarcinomas in male, Copenhagen rats. AIN-93G diets containing these micronutrients were prefed for 4 to 6 weeks prior to tumor implantation by subcutaneous injection. Tumors were allowed to grow for ∼18 weeks. Across diet groups, methylselenocysteine consumption decreased final tumor area (P = 0.003), tumor weight (P = 0.003), and the tumor weight/body weight ratio (P = 0.003), but lycopene and γ-tocopherol consumption intake did not alter any of these measures. There were no significant interactions among nutrient combinations on tumor growth. Methylselenocysteine consumption also led to small, but significant decreases in body weight (P = 0.007), food intake (P = 0.012), and body weight gain/food intake ratio (P = 0.022). However, neither body weight nor gain/food intake ratio was correlated with tumor weight. Methylselenocysteine, lycopene, and γ-tocopherol consumed alone and in combination did not alter serum testosterone or dihydrotestosterone concentrations; tumor proliferation or apoptosis rates. In addition, the diets also did not alter tumor or prostate androgen receptor, probasin, selenoprotein 15, selenoprotein P, or selenium binding protein 2 mRNA expression. However, using castration and finasteride-treated tissues from a previous study, we found that androgen ablation altered expression of these selenium-associated proteins.
Conclusions
Of the three micronutrients tested, only methylselenocysteine consumption reduced growth of transplantable Dunning R3327-H prostate tumors, albeit through an unresolved mechanism.
Introduction
Prostate cancer is the most frequently diagnosed male cancer in the United States, accounting for about one quarter of all male cases in 2009 [1]. Selenium, vitamin E, and lycopene, the red-pigmented carotenoid found primarily in tomatoes, are among the most commonly used supplements by men diagnosed with prostate cancer [2], [3]. Despite their use by prostate cancer patients, it has not been established whether supplementation of these micronutrients, alone or in combination, results in improved outcomes.
Before embarking on large clinical intervention studies, it is often best to first exhaust the use of cell culture and animal models. This has been exemplified by the recent early termination of SELECT, a large clinical intervention study using selenomethionine and all-rac-α-tocopherol acetate alone and in combination for prostate cancer risk reduction, because of the apparent lack of efficacy [4]. Selenomethionine and α-tocopherol have been shown to be only marginally effective in prostate cancer animal models [5]–[9].
An additional consideration in developing natural supplements for cancer prevention or therapy is the selection of the chemical form of the test compounds. Selenomethionine is less effective than methylselenocysteine at decreasing cancer incidence and/or progression in breast [10], [11], colon [10], [12], and prostate cancer models [5], [7], [8], [13]. For vitamin E, epidemiological [14]–[17], in vitro [18]–[20], and animal studies [5], [6], [21]–[24] are more supportive of γ-tocopherol than α-tocopherol for decreasing prostate cancer risk, cell proliferation, or tumor development or growth. Likewise, we reported that lycopene alone is not as effective as 10% tomato powder at decreasing the development or progression of prostate cancer in two animal models [25], [26]. Although these compounds have not been particularly effective when supplemented alone, compelling evidence supports their use in combination [21], [27]–[31].
On the basis of this notion of combinatorial approaches to reduce tumor growth, we tested the ability of lycopene, methylselenocysteine, and γ-tocopherol alone and in combination to decrease the growth of androgen-dependent Dunning R-3327H rat prostate adenocarcinomas. The Dunning R-3327H model is a transplantable tumor model that originated from a spontaneous tumor in a Copenhagen rat [32]. It is an appropriate model for this study because it is a slow-growing, nonmetastatic, androgen-responsive [32], [33] tumor that responds to dietary interventions [26], [34]. We hypothesized that combinations of these micronutrients would additively or synergistically decrease tumor area and weight.
Results
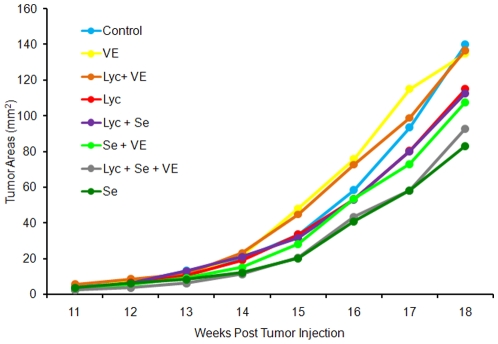
The following abbreviations are used to represent the diet groups: lycopene, Lyc; selenium, Se; Vitamin E, VE; Lyc + Se; Lyc + VE; Lyc + Se + VE. Tumor incidence was greater than 99%, with only one rat injection site not developing a tumor (Lyc + Se). This site was not included in the tumor area or tumor weight calculations. Selenium consumption led to a significant decrease in final tumor area (P = 0.003, Figure 1) and tumor weight (P = 0.003, Table 1). Neither lycopene nor vitamin E consumption altered final tumor area or tumor weight, and no nutrient combination interactions significantly influenced tumor growth.
Figure 1. Dunning R3327-H tumor area (n = 26 to 38).
Selenium consumption led to a significant decrease in tumor area (P = 0.003), but vitamin E and lycopene consumption did not alter tumor areas.
Table 1. Final body weight, food intake, gain/food intake ratio, tumor weight, and tumor weight/final body weight ratio1.
| Group | Final Body Weight (g) | Food Intake (g/d) | Gain/Food intake Ratio (g gained/g total food intake) ×1000 | Tumor Weight (g) | Tumor Weight/Final Body Weight Ratio ×10000 |
| Control | 360±7 (19) | 16.7±0.3 (19) | 70.7±2.2 (19) | 1.26±0.16 (38) | 34.0±4.3 (38) |
| VE2 | 365±9 (14) | 16.6±0.4 (15) | 70.8±2.3 (14) | 1.43±0.19 (27) | 38.4±5.2 (27) |
| Lyc + VE | 364±5 (14) | 16.6±0.4 (14) | 72.1±2.3 (14) | 1.52±0.26 (28) | 40.0±6.8 (28) |
| Lyc | 364±11 (14) | 16.7±0.3 (14) | 72.4±3.6 (14) | 1.13±0.16 (30) | 31.2±4.6 (30) |
| Lyc + Se | 343±8 (14) | 16.2±0.2 (14) | 68.8±2.9 (14) | 0.96±0.14 (27) | 26.8±3.9 (27) |
| Se + VE | 359±10 (14) | 16.6±0.3 (14) | 68.9±2.4 (14) | 1.21±0.20 (27) | 32.8±5.3 (27) |
| Lyc + Se + VE | 338±7 (14) | 16.0±0.3 (15) | 64.6±1.8 (15) | 0.80±0.10 (29) | 22.4±2.7 (29) |
| Se | 347±9 (14) | 16.1±0.4 (14) | 67±2.7 (14) | 0.80±0.11 (26) | 21.9±3.1 (26) |
Data are means ± SEM, ( ) sample size, selenium consumption resulted in a significant decrease in final body weight (P = 0.007), food intake (P = 0.012), gain/food intake ratio (P = 0.022), tumor weight (P = 0.003), and tumor weight/body weight ratio (P = 0.003).
Lyc, lycopene; Se, selenium; VE, vitamin E.
Diets were well accepted and consumed; nevertheless, selenium consumption led to a significant decrease in final body weight (P = 0.007, Table 1). Food intake varied by less than 1 g/day between diet groups (Table 1), but selenium consumption was associated with a small, yet significant, decrease in daily food intake (P = 0.012) and gain/food intake ratio ([body weight gained/total food intake] ×1000, P = 0.022, Table 1). To normalize tumor weight for body weight, we calculated the tumor weight/body weight ratio. This measure had the same P-value (P = 0.003) as the P-value for tumor weight alone. The correlations between body weight and tumor weight (r = 0.12) and gain/food intake ratio and tumor weight (r = 0.09) were not significant.
We used high performance liquid chromatography (HPLC) to measure hepatic lycopene, α-tocopherol, and γ-tocopherol concentrations and neutron activation analysis to quantify selenium concentrations to verify that supplementation was effective and whether nutrient accumulation interactions had occurred. All diets contained 66 mg/kg diet α-tocopherol, γ-tocopherol-supplemented diets contained 195 mg/kg diet γ-tocopherol, and lycopene-supplemented diets contained 291 mg/kg diet lycopene. Hepatic lycopene concentrations in the Lyc + VE group were increased compared with Lyc alone (Table 2, P<0.05). There was no difference in hepatic γ-tocopherol concentrations between vitamin E-supplemented groups or in α-tocopherol among all dietary groups. Lycopene and γ-tocopherol were not detected in nonsupplemented groups (Table 2). Neutron activation analysis showed that selenium-supplemented diets contained 1.03 mg/kg diet, whereas nonsupplemented diets contained 0.09 mg/kg diet selenium. Selenium supplementation increased hepatic selenium concentrations (Table 2, P<0.05), but there were no differences between selenium-supplemented groups.
Table 2. Hepatic lycopene, γ-tocopherol, α-tocopherol, and selenium levels1.
| Group | Total2 Lycopene (µg/g) | γ-tocopherol3 (µg/g) | α-tocopherol (µg/g) | Selenium (µg/g) |
| Control | ND4 | ND | 76±4 | 0.83±0.03 |
| VE5 | ND | 5.5±0.6 | 70±4 | 0.85±0.02 |
| Lyc + VE | 203±13* | 6.1±1.1 | 77±7 | 0.79±0.06 |
| Lyc | 148±20 | ND | 72±3 | 0.77±0.06 |
| Lyc + Se | 171±9 | ND | 78±7 | 1.15±0.06** |
| Se + VE | ND | 6.4±2.0 | 64±5 | 1.16±0.02** |
| Lyc + Se + VE | 149±13 | 3.9±0.7 | 72±3 | 1.12±0.04** |
| Se | ND | ND | 67±5 | 1.17±0.03** |
Data are means ± SEM (n = 6),
P<0.05 vs. Lyc group,
P<0.05 vs. control group.
All-trans + cis isomers.
Limit of detection 0.6 µg/g.
Not detectable.
Lyc, lycopene; Se, selenium; VE, vitamin E.
Because of the importance of androgens in stimulating the growth of Dunning R3327-H tumors, we evaluated whether selenium consumption altered serum testosterone or dihydrotestosterone concentrations. There were no differences in either serum androgen among dietary groups (Table 3). Other potential mechanisms for decreased tumor growth with selenium consumption include altered tumor proliferation and/or apoptotic rates. We used immunohistochemistry to calculate a tumor proliferation index and count tumor apoptotic cells; however, there were no significant differences in these measures among dietary groups (Table 3). We next measured the activity of the selenoenzyme, glutathione peroxidase, to determine whether selenium consumption induced this enzyme in the prostate and/or tumor. We found no difference in glutathione peroxidase activity in the prostate or tumor with selenium consumption compared with animals fed the control diet (data not shown).
Table 3. Serum testosterone, dihydrotestosterone levels, tumor proliferation index, and apoptotic cell numbers1.
| Diet Group | Serum Testosterone (ng/mL) | Serum Dihydrotestosterone (pg/mL) | Tumor Proliferation Index (%) | Tumor Apoptotic Cell Number |
| Control | 1.4±0.2 (17) | 88±9 (14) | 45.2±4.7 (10) | 8.2±0.9 (20) |
| VE2 | 1.2±0.1 (13) | 81±8 (13) | 44.2±4.8 (10) | 9.3±1.1 (20) |
| Lyc + VE | 1.1±0.1 (14) | 83±8 (14) | 44.9±3.1 (10) | 7.5±0.9 (20) |
| Lyc | 1.2±0.1 (11) | 81±10 (12) | 43.7±5.5 (10) | 9.6±0.9 (20) |
| Lyc + Se | 1.4±0.1 (10) | 86±6 (12) | 50.1±4.5 (10) | 7.9±0.6 (20) |
| Se + VE | 1.1 ±0.1 (14) | 80±8 (14) | 47.6±5.0 (10) | 10.4±1.0 (20) |
| Lyc + Se + VE | 1.3±0.1 (13) | 81±7 (15) | 54.2±3.7 (10) | 7.3±0.7 (20) |
| Se | 1.1±0.1 (12) | 75±8 (13) | 46.7±4.4 (10) | 7.6±0.6 (20) |
Data are means ± SEM, ( ) sample size, no significant differences for any of these parameters.
Lyc, lycopene; Se, selenium; VE, vitamin E.
Selenium treatment, or consumption, has been reported to alter androgen receptor signaling or expression [35]–[45]. Thus, we measured androgen receptor and probasin mRNA expression in the normal prostate and tumor of all dietary groups. Probasin (prostate basic protein) is a prostate-specific, androgen-response gene [46]. There was no difference in androgen receptor or probasin mRNA expression between diet groups in either tissue (data not shown).
We next examined whether selenium consumption altered the mRNA expression of three selenium-associated proteins: SepP, Sep15, and SBP2. We selected these selenium-associated proteins for two reasons. First, their expression has been reported to decrease in prostate cancer tissue and/or cell lines compared with normal tissue or less-advanced prostate cancer cell lines [47]–[49]. In addition, their expression is altered by androgens [47], [50]–[54]. Nonetheless, there was no difference in SepP, Sep15, and SBP2 mRNA expression between diet groups in either the prostate or the tumor (data not shown).
Because we did not find differences in SepP, Sep15, and SBP2 mRNA expression with selenium consumption, we measured mRNA expression in prostates and tumors from finasteride-treated and castrated rats from a previous study [26]. This was done to ensure that the expression of SepP, Sep15, and SBP2 was altered by androgens as reported previously [47], [50], [52]–[54]. Finasteride is a 5α-reductase inhibitor that prevents the conversion of testosterone to dihydrotestosterone, making it a weak androgen ablation therapy. In the prostate, castration decreased prostate Sep15 levels (P = 0.0003, Table 4), whereas finasteride treatment decreased prostate SepP expression (P = 0.008, Table 4). In the prostate, SBP2 expression was increased by both finasteride treatment (P = 0.002, Table 4) and castration (P = 0.008, Table 4). In the tumor, castration decreased Sep15 expression levels (P = 0.003, Table 4), and there were no alterations in SBP or SepP expression levels or effects of finasteride treatment.
Table 4. Prostate and tumor selenoprotein 15 (Sep15), selenoprotein P (SepP), and selenium binding protein 2 (SBP2) mRNA expression in castrated and finasteride treated rats1.
| Prostate | Tumor | |||||
| Group | Sep15 (n = 5–6) | SepP (n = 5–6) | SBP2 (n = 5–6) | Sep15 (n = 11–13) | SepP (n = 11–13) | SBP2 (n = 10–13) |
| Control (Cas) | 1.0±0.1 | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 |
| Castrated | 0.4±0.1** | 0.9±0.1 | 2.0±0.1** | 0.7±0.1** | 1.1±0.1 | 1.1±0.1 |
| Control (Fin) | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 |
| Finasteride | 0.8±0.1 | 0.6±0.1* | 1.8±0.2* | 1.0±0.1 | 1.2±0.1 | 0.8±0.1 |
Values are means ± SEM, asterisks indicate significant differences compared with the corresponding control,
P<0.05,
P<0.01.
Discussion
The major finding of this study was that dietary methylselenocysteine, but not lycopene or γ-tocopherol, reduced prostate Dunning R3327-H adenocarcinoma growth in male Copenhagen rats. Contrary to our hypothesis and others' findings [21], [27]–[31], we did not observe additive or synergistic reductions in tumor growth with combinations of the different micronutrients. The ineffectiveness of lycopene alone is, in general, consistent with our [25], [26] and others' findings [21], [22], [55] in rodent prostate cancer models. Unlike the inhibition seen previously in transgenic prostate cancer models [23], [24], γ-tocopherol did not inhibit tumor growth in the present study. These differences may indicate that γ-tocopherol prevents development of prostate cancer, which cannot be investigated in this model, rather than inhibits growth of prostate tumors.
The dietary level of lycopene is the same as our previous rat prostate cancer studies [25], [26], and was originally selected to yield lycopene tissue levels similar to what occurs in humans [56], [57]. We originally set out to supplement the diets with 2 mg Se/kg diet because in breast cancer models this level was able to decrease cancer incidence by 50% and did not result in adverse outcomes, such as decreased food intake or body weights [10]. However, the diet analysis revealed that the diet contained 1 mg Se/kg diet. For γ-tocopherol, we chose 200 mg γ-tocopherol/kg diet based on the finding that 150 mg γ-tocopherol/kg diet increased both α-tocopherol and γ-tocopherol tissue levels in vitamin E deficient rats [58].
The small but significant decreases in body weight, food intake, and gain/food intake ratio with methylselenocysteine consumption was unexpected. Of 20 publications identified [8], [12], [13], [59]–[75], only one [13], which fed 1 to 3 mg Se/kg diet as methylselenocysteine, or vegetable powder that accumulates this compound, for ≥10 weeks, reported a significant decrease in body weight. However, the significance of selenium consumption's reduction in tumor weight was not altered when we controlled for body weight by calculating the tumor/body weight ratio. Further support is provided by correlations between final body weight and tumor weight or gain/food intake ratio and tumor weight that were not statistically significant. Overall, we cannot rule out that decreased body weight, food intake, gain/food intake ratio, or cytoxic effects of selenium may have played a small role in the decrease in tumor growth.
For lycopene, selenium, and vitamin E status, we measured hepatic concentrations, which are a good indicator of prostate and tumor lycopene [26] and selenium concentrations [8] as well as overall body status. Selenium consumption resulted in a 42% increase in hepatic selenium concentrations, similar to what has been reported previously [8]. There was no increase in hepatic α-tocopherol concentrations with γ-tocopherol supplementation as reported previously [58]. Similar to our results, a recent publication also found no alteration in plasma α-tocopherol concentrations in transgenic male rats fed γ-tocopherol [24]. We found a significant 37% increase in hepatic lycopene concentrations with γ-tocopherol consumption that we believe may be due to the antioxidant action of γ-tocopherol, which could prevent oxidation of lycopene. However, hepatic lycopene concentrations were not increased in the Lyc + Se + VE group compared with the Lyc group. More research on combinations of these nutrients is needed to characterize these interactions.
Despite the reduction in tumor weight and final tumor area with selenium consumption, tumor proliferation or apoptosis rates were unaltered. The slopes of the increase in tumor area from week 17 to week 18 (Figure 1) were similar among all diet groups, indicating similar growth rates at the end of the study. Thus, it is possible that if we had terminated the study earlier, when growth rates were different, we may have found differences in tumor proliferation and apoptosis rates. Selenium consumption also did not alter glutathione peroxidase activity in the prostate or tumor, similar to previous findings in the liver and colon of mice [76]. To the best of our knowledge, we are the first to determine that selenium consumption does not alter glutathione peroxidase activity in the prostate or prostate tumors.Selenium consumption did not alter androgen receptor, probasin, SBP2, Sep15, or SepP prostate or tumor mRNA expression. Our finding of no alteration of androgen receptor expression with methylselenocysteine consumption differs from consumption and intraperitoneal injection of methylselenocysteine that decreased androgen receptor expression in the rat prostate [45] and LNCaP tumor xenographs in nude mice [44], respectively. The reason for the difference in results is not clear but could be related to our lower selenium dose, animal model, and/or method of gene expression measurement. To the best of our knowledge, we are the first to determine whether selenium consumption alters expression of SBP2, Sep15, and SepP in the prostate or prostate tumors. There are two very similar SBPs in mice, SBP1 and SBP2 [77], but in both rats and humans, only one SBP has been identified. The human gene is SBP1 [78], and the rat gene is SBP2 [79]. Our finding of no differences in SBP2 with selenium is in agreement with the lack of an effect of selenium consumption on mouse hepatic SBP1 protein levels [80]. However, it differs from the increased SBP1 expression with methylselenocysteine treatment of normal human ovarian surface cells in vitro and decreased expression in three of the four human ovarian cancer cell lines [51]. Our finding that Sep15 expression was not altered by selenium consumption or deficiency is consistent with previous studies in which mouse hepatic Sep15 protein [76], [81] and mRNA levels [81], [82] were not altered with selenium consumption or deficiency. We did not find an alteration in SepP in either tissue, and hepatic SepP expression levels previously were reported to be decreased in selenium-deficient rats [83], but not in the colon of selenium-deficient mice[82].
In castrated or finasteride-treated rats, prostate SBP2 mRNA expression was increased. This is in agreement with work investigating the effects of dihydrotestorone treatments of prostate cancer cells in culture [47], [50]. In contrast, neither castration nor finasteride treatment altered tumor SBP2 expression. Castration significantly decreased prostate and tumor Sep15 expression similar to the decrease in rat ventral prostate Sep15 expression following castration reported previously [52]. It should be noted that this was the only gene altered by castration in both the prostate and tumor. Finasteride treatment decreased prostate SepP expression. Previously, castration was reported to decrease kidney but not hepatic SepP expression in mice [53]. In addition, in LNCaP cells, in vitro synthetic androgen R1881treatment increased SepP expression [54]. Thus, it appears that SepP expression is stimulated by androgens. Overall, Dunning tumor gene expression seemed to be less responsive to androgen ablation than prostate gene expression. This may be due to the location of the tumor or its altered physiology.
In conclusion, only methylselenocysteine consumption decreased tumor weight and final tumor area, and there was no additional benefit of consuming lycopene and/or γ-tocopherol. Methylselenocysteine consumption did not alter serum androgens; tumor proliferation rates; tumor apoptosis rates; glutathione peroxidase activity; or androgen receptor, probasin, Sep15, SepP, or SBP2 expression. Thus, another mechanism of action for selenium's ability to decrease tumor growth is yet to be elucidated. Castration decreased prostate and tumor Sep15 expression and increased prostate SBP2 expression. Finasteride treatment decreased prostate SepP expression and increased prostate SBP2 expression.
The SELECT results show the importance of conducting preclinical animal models and publishing those results even if nonsignificant effects are seen [84], [85]. Evidence in preclinical models suggests that all forms of selenium (selenomethionine, selenite, etc.) should not be lumped together because of their varied efficacy. Instead, studies should be interpreted according to what selenium compound was administered with the current study adds to the methylselenocysteine and prostate cancer literature [86]. Our lycopene and/or γ-tocopherol results add to growing evidence suggesting that isolated nutrients, alone or in combination, may not be an effective strategy for decreasing tumor growth [85], [87], [88].
Materials and Methods
Ethics Statement
The University of Illinois Laboratory Animal Care Advisory Committee approved all animal procedures.
Experimental Diets
Study rats were fed the control diet for 1 week after receipt and then were randomized into one of eight AIN-93G [89] based powder study diet groups (Table 5) for 4 to 6 weeks prior to tumor implantation. Diets were balanced for protein, fat, energy, and fiber and stored at 4°C in the dark. All diets were provided ad libitum. Diets were replaced and food intakes calculated every 48 h. Fresh diets were prepared monthly (all diet ingredients from Harlan Teklad, Madison, WI, unless otherwise noted).
Table 5. AIN-93G-based lycopene, selenium, and vitamin E diet formulations.
| Diets Composition (g) | Control | Lyc1 | Se | VE | Lyc + Se | Lyc + VE | Se + VE | Lyc + Se + VE |
| Cornstarch | 395 | 395 | 385 | 395 | 385 | 395 | 385 | 385 |
| Casein | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Maltodextrin | 132 | 132 | 132 | 132 | 132 | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Soybean Oil2 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| Cellulose | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Mineral Mix | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin Mix3 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| L-Cystine | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Lyc Beadlets4 | - | 2.5 | - | - | 2.5 | 2.5 | - | 2.5 |
| Placebo Beadlets | 2.5 | - | 2.5 | 2.5 | - | - | 2.5 | - |
| γ-Tocopherol | - | - | - | 0.2 | - | 0.2 | 0.2 | 0.2 |
| Se Premix5 | - | - | 10 | - | 10 | - | 10 | 10 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
Lyc, lycopene; Se, selenium; VE, vitamin E.
Tocopherol-stripped.
27 mg/kg diet as all-rac-α-tocopherol acetate.
10% lycopene.
200 mg/kg of selenium in cornstarch as methylselenocysteine.
Tocopherol-stripped soybean oil (Dyets, Inc., Bethlehem, PA) was used to limit exogenous, nonsupplemented tocopherol levels. In addition, the vitamin mix (Harlan Teklad TD.05521) was formulated to provide the National Research Council requirement for rats (27 mg/kg diet as all-rac-α-tocopherol acetate) [90] instead of the 75 mg/kg diet normally provided by AIN93-G diets [89]. Vitamin E was supplemented as γ-tocopherol (D-Gamma Tocopherol 90, Eisai Food & Chemical Co., Ltd., Tokyo, Japan) at 200 mg/kg diet in VE groups. Lycopene was added to diets in the form of 10% lycopene beadlets (redivivoTM, DSM, Basel, Switzerland) to deliver 250 mg/kg diet as we have done previously [25], [26]. Equivalent amounts of placebo beadlets (DSM) were used in diets not containing lycopene. Selenium was supplemented in the form of methylselenocysteine (Se-Methylseleno-L-cysteine, PharmaSe, Lubbock, TX) at 1 mg/kg diet of elemental selenium.
Rats
One hundred and twenty-six ≈100 gram male Copenhagen rats (Cop 2331; Harlan, Indianapolis, IN) were individually housed in wire-bottomed cages. Rats were monitored daily and weighed twice weekly. Two control rats were euthanized prior to study completion because of high tumor burden. Five other rats were euthanized prior to study completion for health reasons unrelated to tumor growth (1 Se, 1 VE, 1 Lyc + Se, 1 Lyc + VE, 1 VE + Se); 119 rats completed the study. The study was carried out in three cohorts (n = 6 or 7) with 6 or 7 control rats per cohort. Cohorts were necessary because the rats could not be purchased at one time. Cohort was used as a covariate in statistical analysis to control for possible differences between cohorts.
Dunning R3327-H Tumor Implantations
Ten ≈150 g male Copenhagen donor rats (Harlan, Indianapolis, IN) had tumor pieces coated in Matrigel™ Basement Membrane Matrix (BD Biosciences, Bedford, MA) subcutaneously implanted in their hind flanks. Tumors were allowed to grow for 15 to 20 weeks and then were harvested for implantation into study rats.
Tumors were sterilely harvested from donor rats by removing the tumor capsule, necrotic areas, and large blood vessels. The remaining tissue was mixed with Hanks Balanced Salt Solution, minced, pushed through a 20 mesh sieve, and placed in a 50 mL centrifuge tube. The solution was centrifuged at 200×g for 10 minutes, and the supernatant was removed. The remaining tumor pellet was mixed with Matrigel™ at a concentration of 100 mg tumor/mL, and 0.2 mL (≈20 mg of tumor) was injected with 19-gauge needles into both rear flanks of study rats. Tumors were allowed to grow for ≈18 weeks until study termination. Tumors were palpated weekly, and tumor length and width were measured with calipers. From these measurements, tumor area was calculated using the formula for area of an ellipse: area = π*(length/2)*(width/2). When a tumor was not found, a zero was recorded for that week. At study termination, rats were anesthetized with CO2, blood was taken via cardiac puncture, tumors were removed and cleaned, and tumor weights were recorded. Tumor and selected tissues were flash frozen in liquid nitrogen and then stored in a −80°C freezer. In addition, a piece of tumor was placed in 10% formalin (Sigma-Aldrich) for 24 to 48 hours before being moved to 70% ethanol.
Hepatic and Diet Lycopene and Vitamin E Quantification
For liver, 0.1 g of tissue was placed into a 50-mL glass centrifuge tube. Ethanol containing 0.1% butylated hydroxytoluene (3.5 mL) was added along with deionized distilled water (1.5 mL) and ascorbic acid (0.25 g). Tocol (Matreya, Pleasant Gap, PA) and β-carotene (Sigma-Aldrich, St. Louis, MO) were added as internal standards for tocopherols and carotenoids, respectively. Saturated potassium hydroxide (1 mL) was then added, and samples were vortexed and saponified at 60°C for 30 min in a water bath. Ascorbic acid was necessary to prevent oxidation of vitamin E during saponification as described previously [91]. Test tubes were then removed from the water bath and placed on ice, and deionized distilled water (2 mL) was added prior to extraction three times with hexane (6 mL). The extracts were dried down in a Speedvac concentrator (model AES1010; Savant, Farmingdale, NY), covered with argon, and stored at −20°C for less than 48 hours before HPLC analysis. The procedure was the same for diet extraction except 0.25 g of diet was extracted, and internal standards were not used. Samples were analyzed by using an isocratic HPLC method described previously with minor modifications [92]. The mobile phase consisted of acetonitrile/dioxane/2-propanol/methanol/triethylamine (800/150/25/25/1) with 200 mM ammonium acetate in the alcohol component. Samples were reconstituted in ethyl acetate: mobile phase (100∶35). The HPLC system consisted of a Varian Prostar 325 UV-Vis Dual Wavelength Detector (Walnut Creek, CA) monitoring 300 nm and 450 nm wavelengths, a Varian 410 Autosampler, Rainin SD-200 Dynamax pumps, a precolumn (Upchurch Scientific, Oak Harbor, WA) packed with ODS C-18 (Alltech Associates, Deerfield, IL), a C-18 Waters Spherisorb 3 µM ODS2 4.6×150 mm column (Milford, MA), and a CERA 250 column cooler (Baldwin Park, CA) set at 16°C for the 25-minute run duration. Our laboratory routinely participates in the National Institutes of Standards in Technology micronutrient proficiency testing program, and our values for lycopene, α-tocopherol, and γ-tocopherol normally are within one standard deviation of the median.
Selenium and Serum Androgen Quantification
Hepatic and diet selenium concentrations were determined by neutron activation analysis [93] at the University of Missouri Research Reactor Center (reported as wet weight concentrations). Serum testosterone and dihydrotestosterone concentrations were determined with DSL-4000 and DSL-9600 radioimmunoassay kits, respectively (Diagnostic Systems Laboratories Inc., Webster, TX) according to the manufacturer's instructions.
Tumor Histology and Immunohistochemistry
At study termination, a piece of Dunning R3327-H tumor was fixed in 10% formalin (Sigma-Aldrich) for 24 to 48 hours before being moved to 70% ethanol. Samples were then processed overnight in a vacuum infiltration processor E300, (Sakura Finetek, Torrance, CA), paraffin embedded by using a Tissue Tek TEC Embedding Center (Sakura Finetek), sectioned at 3 µM by using a HM315 Microm Microtome, and transferred to positively charged barrier slides (BioGenex, San Ramon, CA). Slides were deparafinized and hydrated by using a Sakura DRS 2000 followed by methanol 3% H2O2 solution for 15 min. To unmask proliferating cell nuclear antigen (PCNA), slides were placed in citrate buffer (pH 6) in a decloaking chamber for 30 seconds at 125°C and 10 seconds at 90°C. The slides were then transferred to a Biogenex i6000 immunostainer, where the slides were blocked for 10 minutes with Power Block™ (BioGenex) then blocked with avidin and biotin for 15 minutes each. Rabbit anti-PCNA antibody (Dako, Carpinteria, CA) was incubated for 30 minutes followed by the DakoCytomation LSAB2 System. The LSAB2 Biotinylated Link was incubated for 15 min, the LSAB2 Streptavidin-HRP label for 15 minutes, followed by 3,3′-diaminobenzidine tetrahydrochloride (BioGenex) for 5 minutes. The slides were then counterstained with Mayer's hematoxylin (BioGenex) for 1 minute. The PCNA images were blindly captured and quantified as described previously [26]. Two representative images per tumor were captured, and a proliferative index percentage, (PCNA positive/total nuclei counter) ×100, was calculated. The proliferative index reported is the mean of two proliferative index percentages per tumor.
Apoptosis was measured with an Apoptag® Peroxidase In Situ Apoptosis Detection Kit S7100 (Chemicon International, Temecula, CA) according to the manufacturer's instructions except that slides were counterstained with Mayer's hematoxylin rather than methyl green. Images were blindly captured and quantified as described previously [26]. Two representative images, without artifacts, from each tumor were quantified by counting the number of nuclei at 400× magnification. The apoptotic cell number reported is the mean of the two apoptotic nuclei values per tumor.
Real-Time PCR
Castration and finasteride tissues were from two separate studies described previously [26]; therefore, controls were needed as a comparison to both groups. Tumors and dorsolateral plus anterior prostate lobes were extracted with the PureLink Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Tumors and dorsolateral prostates from the Lyc, Se, and VE groups were extracted using Trizol® reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. After extraction, the sample was treated with DNase (DNase I, New England Biolabs, Ipswich, MA) following the manufacturer's typical reaction instructions except 20 µg of RNA and four units of DNAse I were used. The RNA was then ethanol precipitated [94] and resuspended in RNase-free water. The RNA was run on an agarose gel to examine RNA integrity, and samples of sufficient quality were used to synthesize cDNA with High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA) and an Eppendorf Mastercycler Gradient (Westbury, NY).
Primers were selected across exon boundaries by using Primer Express software. Primers (MWG, Huntsville, AL) were validated to ensure efficiency and amplification of a single amplicon. The primers selected were: androgen receptor forward AGAACTACTCCGGACCTTATGGG, reverse TGGTCCCTGGTACTGTCCAAAC; probasin forward AGAAATGACGTTACACGAGTGGC, reverse CAGTTGCCTGCCTTTCGC; SepP forward AGCCAGCTGATACCTGTGCC, reverse CGCAGGTCTTCCAATCTGGA; Sep15 forward AACCCAAACTGTTCAGAGGTCTACA, reverse GGTCTGAGCCTCGAACATACTTG; and SBP2 forward GCCTCACTGGGCAGATCTTC, reverse CAGAGCCTCCTTTAACAATGCTG.
The rRNA L7a was used as an internal control by using previously published sequences [95]. We verified equivalent expression between diet and treatment groups in both tissues examined. Real-time PCR was performed with SYBR Green Mastermix (Applied Biosystems), a 7900 Fast HT Real-Time PCR machine (Applied Biosystems), and 4 ng prostate or tumor cDNA template according to the manufacturer's instructions. The relative mRNA abundance was determined by using the comparative critical threshold method and is expressed relative to the control diet group [96].
Gluathione Peroxidase Activity
Frozen dorsolateral prostate and tumor samples were resuspended in cold sodium phosphate buffer (0.1M, pH 7.0) and lysed by sonication. Total glutathione peroxidase activity was determined using a standard coupled spectrophometric method based on the consumption of reduced NADPH measured spectrophotometrically by absorbance at 339 nm in a reaction containing reduced glutathione, glutathione reductase, tissue lysates, and hydrogen peroxide as described elsewhere [97].
Statistics
Tumor weight; final tumor area; final body weight; daily food intake; gain/food intake ratio; tumor weigh/body weight ratio; proliferation indexes; apoptotic cell numbers; and Lyc, Se, and VE tumor and prostate gene expression were analyzed by using dummy-coded multiple linear regression to look for the effects of lycopene, selenium, and vitamin E consumption and interactions among these nutrients [98], [99]. Cohort was used as a covariate (effects coded) but was retained only in the models for final tumor area, daily food intake, and gain/food intake ratio. Hierarchical analysis revealed that covariate interaction terms and treatment interaction terms did not account for a significant amount of variance; thus, these terms were removed from all the models. For tumor weight; final body weight; tumor weight/body weight ratio; proliferation indexes; apoptotic cell numbers; and Lyc, Se, and VE gene expression, the final models contained only the three main treatment variables (Lyc, Se, and VE). The natural logs of both tumor area and tumor weight were used to correct violations of model assumptions. Tumor weight analysis with proc univariate in SAS identified five very large, outlying tumors (greater than 1.5× interquartile range). These five tumors (2 Se, 1 VE, 1 Se + VE, 1 Lyc + Se + VE) were excluded from tumor weight and final tumor area analysis. One rat (Lyc) was excluded from gain/food intake ratio analysis because of weight loss at the end of the study. Serum testosterone, serum dihydrotestosterone, hepatic lycopene, hepatic vitamin E, and hepatic selenium concentrations were analyzed using ANOVA with Dunnett's test. Correlations of final body weight with tumor weight and final body weight with gain/food intake ratio were performed using the proc corr command in SAS. Castration and finasteride treatment expression levels were analyzed using two-sample t-tests. Natural logs of a number of castration and finasteride treatment gene expression levels were used to correct for assumption violations. When this transformation did not correct assumption violations, the Wilcoxon Rank Exact Sum test [100] was used. All statistics were performed using SAS 9.1 (Carey, NC), and P<0.05 was considered significant.
Acknowledgments
We thank Dr. Karin Wertz at DSM for providing the lycopene and placebo beadlets; Mr. Toyoh Banno Eisai Food & Chemical Co., Ltd., for providing the γ-tocopherol; Dr. John Isaacs for providing the Dunning R3327-H tumor; Dr. Ronald Weigel for his consultation about the statistical analysis; and Sung Eun Choi, Dave Woessner, and Alex Nham for their technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Supported in part by American Institute for Cancer Research (http://www.aicr.org/site/PageServer) Grant 05A021 and Jonathan Baldwin Turner Fellowships (to B.L.L and K.C–A) from the College of Agricultural, Consumer and Environmental Sciences at the University of Illinois at Urbana-Champaign. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Cancer Society. Cancer facts and figures. 2009.
- 2.Boon H, Westlake K, Stewart M, Gray R, Fleshner N, et al. Use of complementary/alternative medicine by men diagnosed with prostate cancer: Prevalence and characteristics. Urology. 2003;62(5):849–853. doi: 10.1016/s0090-4295(03)00668-x. [DOI] [PubMed] [Google Scholar]
- 3.Cheetham PJ, Le Monnier KJ, Brewster SF. Attitudes and use of alternative therapies in UK prostate cancer patients-isn't it time we were in the know? Prostate Cancer Prostatic Dis. 2001;4(4):235–241. doi: 10.1038/sj.pcan.4500536. [DOI] [PubMed] [Google Scholar]
- 4.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick DL, Rao KV. Chemoprevention of hormone-dependent prostate cancer in the wistar-unilever rat. Eur Urol. 1999;35(5-6):464–467. doi: 10.1159/000019880. [DOI] [PubMed] [Google Scholar]
- 6.Fleshner N, Fair WR, Huryk R, Heston WD. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J Urol. 1999;161(5):1651–1654. [PubMed] [Google Scholar]
- 7.Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171(2):907–910. doi: 10.1097/01.ju.0000092859.16817.8e. [DOI] [PubMed] [Google Scholar]
- 8.Li GX, Lee HJ, Wang Z, Hu H, Liao JD, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29(5):1005–1012. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormick D, Rao KVN, Johnson W, Bosland M, Lubet R, et al. Null activity of selenium and vitamin E as cancer chemopreventive agents in the rat prostate. Cancer Prevention Research. 2010. [DOI] [PMC free article] [PubMed]
- 10.Whanger PD. Selenium and its relationship to cancer: An update. Br J Nutr. 2004;91(1):11–28. doi: 10.1079/bjn20031015. [DOI] [PubMed] [Google Scholar]
- 11.Medina D, Thompson H, Ganther H, Ip C. Se-methylselenocysteine: A new compound for chemoprevention of breast cancer. Nutr Cancer. 2001;40(1):12–17. doi: 10.1207/S15327914NC401_5. [DOI] [PubMed] [Google Scholar]
- 12.Finley JW, Davis CD. Selenium (se) from high-selenium broccoli is utilized differently than selenite, selenate and selenomethionine, but is more effective in inhibiting colon carcinogenesis. Biofactors. 2001;14(1-4):191–196. doi: 10.1002/biof.5520140124. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Bonorden MJ, Li G, Lee H, Hu H, et al. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prevention Research. 2009;2(5):484–495. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92(24):2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 15.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, et al. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157(4):335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein SJ, Wright ME, Pietinen P, King I, Tan C, et al. Serum alpha-tocopherol and gamma-tocopherol in relation to prostate cancer risk in a prospective study. J Natl Cancer Inst. 2005;97(5):396–399. doi: 10.1093/jnci/dji045. [DOI] [PubMed] [Google Scholar]
- 17.Wright ME, Weinstein SJ, Lawson KA, Albanes D, Subar AF, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- 18.Galli F, Stabile AM, Betti M, Conte C, Pistilli A, et al. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys. 2004;423(1):97–102. doi: 10.1016/j.abb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16(14):1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. Gamma-tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101(51):17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limpens J, Schroder FH, de Ridder CM, Bolder CA, Wildhagen MF, et al. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J Nutr. 2006;136(5):1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- 22.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, et al. Lycopene and vitamin E interfere with autocrine/paracrine loops in the dunning prostate cancer model. FASEB J. 2004;18(9):1019–1021. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 23.Barve A, Khor TO, Nair S, Reuhl K, Suh N, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. International Journal of Cancer. 2009;124(7):1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi S, Takeshita K, Seeni A, Sugiura S, Tang M, et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate. 2009;69(6):644–651. doi: 10.1002/pros.20915. [DOI] [PubMed] [Google Scholar]
- 25.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, et al. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95(21):1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 26.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, et al. Combinations of tomato and broccoli enhance antitumor activity in dunning R3327-H prostate adenocarcinomas. Cancer Res. 2007;67(2):836–843. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 27.Venkateswaran V, Fleshner NE, Klotz LH. Synergistic effect of vitamin E and selenium in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2004;7(1):54–56. doi: 10.1038/sj.pcan.4500707. [DOI] [PubMed] [Google Scholar]
- 28.Zu K, Ip C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003;63(20):6988–6995. [PubMed] [Google Scholar]
- 29.Ni J, Chen M, Zhang Y, Li R, Huang J, et al. Vitamin E succinate inhibits human prostate cancer cell growth via modulating cell cycle regulatory machinery. Biochem Biophys Res Commun. 2003;300(2):357–363. doi: 10.1016/s0006-291x(02)02851-6. [DOI] [PubMed] [Google Scholar]
- 30.Venkateswaran V, Fleshner NE, Sugar LM, Klotz LH. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64(16):5891–5896. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 31.Venkateswaran V, Klotz L, Ramani M, Sugar L, Jacob L, et al. A combination of micronutrients is beneficial in reducing the incidence of prostate cancer and increasing survival in the lady transgenic model. Cancer Prevention Research. 2009;2(5):473–483. doi: 10.1158/1940-6207.CAPR-08-0124. [DOI] [PubMed] [Google Scholar]
- 32.Tennant TR, Kim H, Sokoloff M, Rinker-Schaeffer CW. The dunning model. Prostate. 2000;43(4):295–302. doi: 10.1002/1097-0045(20000601)43:4<295::aid-pros9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986;9(3):261–281. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- 34.Clinton SK, Palmer SS, Spriggs CE, Visek WJ. Growth of dunning transplantable prostate adenocarcinomas in rats fed diets with various fat contents. J Nutr. 1988;118(7):908–914. doi: 10.1093/jn/118.7.908. [DOI] [PubMed] [Google Scholar]
- 35.Cho SD, Jiang C, Malewicz B, Dong Y, Young CY, et al. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther. 2004;3(5):605–611. [PubMed] [Google Scholar]
- 36.Chun JY, Nadiminty N, Lee SO, Onate SA, Lou W, et al. Mechanisms of selenium down-regulation of androgen receptor signaling in prostate cancer. Mol Cancer Ther. 2006;5(4):913–918. doi: 10.1158/1535-7163.MCT-05-0389. [DOI] [PubMed] [Google Scholar]
- 37.Marshall JR, Sakr W, Wood D, Berry D, Tangen C, et al. Design and progress of a trial of selenium to prevent prostate cancer among men with high-grade prostatic intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1479–1484. doi: 10.1158/1055-9965.EPI-05-0585. [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Zhang H, Gao AC, Marshall JR, Ip C. Androgen receptor signaling intensity is a key factor in determining the sensitivity of prostate cancer cells to selenium inhibition of growth and cancer-specific biomarkers. Mol Cancer Ther. 2005;4(7):1047–1055. doi: 10.1158/1535-7163.MCT-05-0124. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y, Lee SO, Zhang H, Marshall J, Gao AC, et al. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64(1):19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 40.Morris JD, Pramanik R, Zhang X, Carey AM, Ragavan N, et al. Selenium- or quercetin-induced retardation of DNA synthesis in primary prostate cells occurs in the presence of a concomitant reduction in androgen-receptor activity. Cancer Lett. 2006;239(1):111–122. doi: 10.1016/j.canlet.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Whitfield ML, Xu T, Botstein D, Brooks JD. Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells. Mol Biol Cell. 2004;15(2):506–519. doi: 10.1091/mbc.E03-07-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Husbeck B, Bhattacharyya RS, Feldman D, Knox SJ. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: Two distinct mechanisms of action. Mol Cancer Ther. 2006;5(8):2078–2085. doi: 10.1158/1535-7163.MCT-06-0056. [DOI] [PubMed] [Google Scholar]
- 43.Pinto JT, Sinha R, Papp K, Facompre ND, Desai D, et al. Differential effects of naturally occurring and synthetic organoselenium compounds on biomarkers in androgen responsive and androgen independent human prostate carcinoma cells. Int J Cancer. 2007;120(7):1410–1417. doi: 10.1002/ijc.22500. [DOI] [PubMed] [Google Scholar]
- 44.Lee SO, Yeon Chun J, Nadiminty N, Trump DL, Ip C, et al. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA). Prostate. 2006;66(10):1070–1075. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 45.Legg RL, Tolman JR, Lovinger CT, Lephart ED, Setchell KD, et al. Diets high in selenium and isoflavones decrease androgen-regulated gene expression in healthy rat dorsolateral prostate. Reproductive Biology and Endocrinology. 2008;6:57. doi: 10.1186/1477-7827-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasper S, Matusik RJ. Rat probasin: Structure and function of an outlier lipocalin. Biochim Biophys Acta. 2000;1482(1-2):249–258. doi: 10.1016/s0167-4838(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Sytkowski AJ. Differential expression and androgen regulation of the human selenium-binding protein gene hSP56 in prostate cancer cells. Cancer Res. 1998;58(14):3150–3153. [PubMed] [Google Scholar]
- 48.Kumaraswamy E, Malykh A, Korotkov KV, Kozyavkin S, Hu Y, et al. Structure-expression relationships of the 15-kDa selenoprotein gene. possible role of the protein in cancer etiology. J Biol Chem. 2000;275(45):35540–35547. doi: 10.1074/jbc.M004014200. [DOI] [PubMed] [Google Scholar]
- 49.Calvo A, Xiao N, Kang J, Best CJ, Leiva I, et al. Alterations in gene expression profiles during prostate cancer progression: Functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002;62(18):5325–5335. [PubMed] [Google Scholar]
- 50.Velasco AM, Gillis KA, Li Y, Brown EL, Sadler TM, et al. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology. 2004;145(8):3913–3924. doi: 10.1210/en.2004-0311. [DOI] [PubMed] [Google Scholar]
- 51.Huang KC, Park DC, Ng SK, Lee JY, Ni X, et al. Selenium binding protein 1 in ovarian cancer. Int J Cancer. 2006;118(10):2433–2440. doi: 10.1002/ijc.21671. [DOI] [PubMed] [Google Scholar]
- 52.Pang ST, Dillner K, Wu X, Pousette A, Norstedt G, et al. Gene expression profiling of androgen deficiency predicts a pathway of prostate apoptosis that involves genes related to oxidative stress. Endocrinology. 2002;143(12):4897–4906. doi: 10.1210/en.2002-220327. [DOI] [PubMed] [Google Scholar]
- 53.Riese C, Michaelis M, Mentrup B, Gotz F, Kohrle J, et al. Selenium-dependent pre- and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology. 2006;147(12):5883–5892. doi: 10.1210/en.2006-0689. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi Y, Hursting SD, Perkins SN, Wang TC, Wang TT. Genistein affects androgen-responsive genes through both androgen- and estrogen-induced signaling pathways. Mol Carcinog. 2006;45(1):18–25. doi: 10.1002/mc.20153. [DOI] [PubMed] [Google Scholar]
- 55.Tang L, Jin T, Zeng X, Wang JS. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J Nutr. 2005;135(2):287–290. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- 56.Boileau TW, Clinton SK, Erdman JW., Jr Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr. 2000;130(6):1613–1618. doi: 10.1093/jn/130.6.1613. [DOI] [PubMed] [Google Scholar]
- 57.Boileau TW, Clinton SK, Zaripheh S, Monaco MH, Donovan SM, et al. Testosterone and food restriction modulate hepatic lycopene isomer concentrations in male F344 rats. J Nutr. 2001;131(6):1746–1752. doi: 10.1093/jn/131.6.1746. [DOI] [PubMed] [Google Scholar]
- 58.Clement M, Bourre JM. Graded dietary levels of RRR-gamma-tocopherol induce a marked increase in the concentrations of alpha- and gamma-tocopherol in nervous tissues, heart, liver and muscle of vitamin-E-deficient rats. Biochim Biophys Acta. 1997;1334(2-3):173–181. doi: 10.1016/s0304-4165(96)00090-6. [DOI] [PubMed] [Google Scholar]
- 59.Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991;51(2):595–600. [PubMed] [Google Scholar]
- 60.Ip C, Lisk DJ, Stoewsand GS. Mammary cancer prevention by regular garlic and selenium-enriched garlic. Nutr Cancer. 1992;17(3):279–286. doi: 10.1080/01635589209514197. [DOI] [PubMed] [Google Scholar]
- 61.Ip C, Ganther HE. Comparison of selenium and sulfur analogs in cancer prevention. Carcinogenesis. 1992;13(7):1167–1170. doi: 10.1093/carcin/13.7.1167. [DOI] [PubMed] [Google Scholar]
- 62.Ip C, Lisk DJ. Enrichment of selenium in allium vegetables for cancer prevention. Carcinogenesis. 1994;15(9):1881–1885. doi: 10.1093/carcin/15.9.1881. [DOI] [PubMed] [Google Scholar]
- 63.Ip C, Lisk DJ. Characterization of tissue selenium profiles and anticarcinogenic responses in rats fed natural sources of selenium-rich products. Carcinogenesis. 1994;15(4):573–576. doi: 10.1093/carcin/15.4.573. [DOI] [PubMed] [Google Scholar]
- 64.Ip C, Lisk DJ. Efficacy of cancer prevention by high-selenium garlic is primarily dependent on the action of selenium. Carcinogenesis. 1995;16(11):2649–2652. doi: 10.1093/carcin/16.11.2649. [DOI] [PubMed] [Google Scholar]
- 65.Ip C, Lisk DJ, Thompson HJ. Selenium-enriched garlic inhibits the early stage but not the late stage of mammary carcinogenesis. Carcinogenesis. 1996;17(9):1979–1982. doi: 10.1093/carcin/17.9.1979. [DOI] [PubMed] [Google Scholar]
- 66.Ip C, Zhu Z, Thompson HJ, Lisk D, Ganther HE. Chemoprevention of mammary cancer with se-allylselenocysteine and other selenoamino acids in the rat. Anticancer Res. 1999;19(4B):2875–2880. [PubMed] [Google Scholar]
- 67.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: Evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60(11):2882–2886. [PubMed] [Google Scholar]
- 68.Ip C, Birringer M, Block E, Kotrebai M, Tyson JF, et al. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J Agric Food Chem. 2000;48(6):2062–2070. doi: 10.1021/jf000051f. [DOI] [PubMed] [Google Scholar]
- 69.Finley JW, Davis CD, Feng Y. Selenium from high selenium broccoli protects rats from colon cancer. J Nutr. 2000;130(9):2384–2389. doi: 10.1093/jn/130.9.2384. [DOI] [PubMed] [Google Scholar]
- 70.Davis CD, Zeng H, Finley JW. Selenium-enriched broccoli decreases intestinal tumorigenesis in multiple intestinal neoplasia mice. J Nutr. 2002;132(2):307–309. doi: 10.1093/jn/132.2.307. [DOI] [PubMed] [Google Scholar]
- 71.Unni E, Kittrell FS, Singh U, Sinha R. Osteopontin is a potential target gene in mouse mammary cancer chemoprevention by se-methylselenocysteine. Breast Cancer Res. 2004;6(5):R586–92. doi: 10.1186/bcr914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, Xie Y, El-Sayed WM, Szakacs JG, Franklin MR, et al. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J Biochem Mol Toxicol. 2005;19(6):396–405. doi: 10.1002/jbt.20105. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Zarbl H. Chemopreventive doses of methylselenocysteine alter circadian rhythm in rat mammary tissue. Cancer Prevention Research. 2008;1(2):119. doi: 10.1158/1940-6207.CAPR-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahn A, Toledo H, Ruz M. Organic and inorganic selenium compounds produce different protein patterns in the blood plasma of rats. Biol Res. 2009;42(2):163. [PubMed] [Google Scholar]
- 75.Mahn A, Toledo H, Ruz M. Dietary supplementation with selenomethylselenocysteine produces a differential proteomic response. J Nutr Biochem. 2009;20(10):791. doi: 10.1016/j.jnutbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136(5):1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 77.Lanfear J, Fleming J, Walker M, Harrison P. Different patterns of regulation of the genes encoding the closely related 56 kDa selenium- and acetaminophen-binding proteins in normal tissues and during carcinogenesis. Carcinogenesis. 1993;14(3):335–340. doi: 10.1093/carcin/14.3.335. [DOI] [PubMed] [Google Scholar]
- 78.Chang PW, Tsui SK, Liew C, Lee CC, Waye MM, et al. Isolation, characterization, and chromosomal mapping of a novel cDNA clone encoding human selenium binding protein. J Cell Biochem. 1997;64(2):217–224. doi: 10.1002/(sici)1097-4644(199702)64:2<217::aid-jcb5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 79.Ishida T, Ishii Y, Yamada H, Oguri K. The induction of hepatic selenium-binding protein by aryl hydrocarbons (ah)-receptor ligands in rats. J Health Sci. 2002;48(1):62–68. [Google Scholar]
- 80.Bansal MP, Mukhopadhyay T, Scott J, Cook RG, Mukhopadhyay R, et al. DNA sequencing of a mouse liver protein that binds selenium: Implications for selenium's mechanism of action in cancer prevention. Carcinogenesis. 1990;11(11):2071–2073. doi: 10.1093/carcin/11.11.2071. [DOI] [PubMed] [Google Scholar]
- 81.Novoselov SV, Calvisi DF, Labunskyy VM, Factor VM, Carlson BA, et al. Selenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic mice. Oncogene. 2005;24(54):8003–8011. doi: 10.1038/sj.onc.1208940. [DOI] [PubMed] [Google Scholar]
- 82.Kipp A, Banning A, van Schothorst E, Mplan C, Schomburg L, et al. Four selenoproteins, protein biosynthesis, and wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Molecular Nutrition Food Research. 2009;53(12):1561. doi: 10.1002/mnfr.200900105. [DOI] [PubMed] [Google Scholar]
- 83.Burk RF, Hill KE. Selenoprotein P. A selenium-rich extracellular glycoprotein. J Nutr. 1994;124(10):1891–1897. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- 84.Bosland MC, McCormick DL. Antioxidant supplementation and cancer prevention. JAMA. 2009;301(18):1877–1878. doi: 10.1001/jama.2009.628. [DOI] [PubMed] [Google Scholar]
- 85.Block KI. Antioxidants: SELECTed out? Integrative Cancer Therapies. 2009;8(1):5–8. doi: 10.1177/1534735409332380. [DOI] [PubMed] [Google Scholar]
- 86.El-Bayoumy K. The negative results of the SELECT study do not necessarily discredit the selenium-cancer prevention hypothesis. Nutr Cancer. 2009;61(3):285–286. doi: 10.1080/01635580902892829. [DOI] [PubMed] [Google Scholar]
- 87.Gann PH. Randomized trials of antioxidant supplementation for cancer prevention: First bias, now chance—next, cause. JAMA. 2009;301(1):102–103. doi: 10.1001/jama.2008.863. [DOI] [PubMed] [Google Scholar]
- 88.Gann PH, Khachik F. Tomatoes or lycopene versus prostate cancer: Is evolution anti-reductionist? J Natl Cancer Inst. 2003;95(21):1563–1565. doi: 10.1093/jnci/djg112. [DOI] [PubMed] [Google Scholar]
- 89.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 90.Council NR. Anonymous Nutrient Requirements of Laboratory Animals. Washington, D.C.: National Academy Press; 1995. Nutrient requirements of the laboratory rat. pp. 11–79. [Google Scholar]
- 91.Vatassery GT, Smith WE, Quach HT. A liquid chromatographic method for the simultaneous determination of alpha-tocopherol and tocopherolquinone in human red blood cells and other biological samples where tocopherol is easily oxidized during sample treatment. Anal Biochem. 1993;214(2):426–430. doi: 10.1006/abio.1993.1518. [DOI] [PubMed] [Google Scholar]
- 92.Craft NE, Furr HC. Improved HPLC analysis of retinol and retinyl esters, tocopherols, and carotenoids in human serum samples for the NHANES. FASEB J. 2004;18:A534. [Google Scholar]
- 93.McKown DM, Morris JS. Rapid measurement of selenium in biological samples using instrumental neutron activation analysis. J Radioanal Chem. 1978;43(2):411–420. [Google Scholar]
- 94.Sambrook J, Russell DW. Cold Springs Harbor: Cold Springs Harbor Laboratory Press; 2001. Molecular cloning a laboratory manual. [Google Scholar]
- 95.Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem. 2004;279(49):50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- 96.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 97.Samuels BL, Murray JL, Cohen MB, Safa AR, Sinha BK, et al. Increased glutathione peroxidase activity in a human sarcoma cell line with inherent doxorubicin resistance. Cancer Res. 1991;51(2):521–527. [PubMed] [Google Scholar]
- 98.Cohen J, Cohen P. Hillsdale: Lawrence Erlbaum Associates, Publishers; 1983. Applied multiple Regression/Correlation analysis for the behavioral sciences. [Google Scholar]
- 99.Weigel RM, Narvaez M. Multiple regression analysis of differential response to treatment in randomized controlled clinical trials. Control Clin Trials. 1991;12(3):378–394. doi: 10.1016/0197-2456(91)90018-h. [DOI] [PubMed] [Google Scholar]
- 100.Narayanan A, Watts D. Exact methods in the NPAR1WAY procedure. 1996.