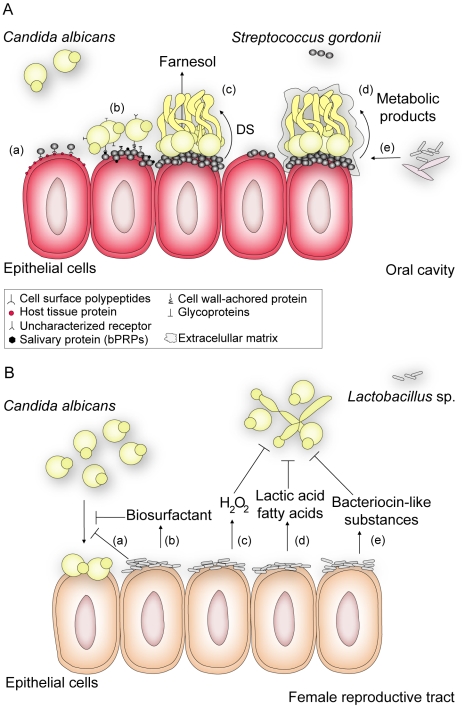
Figure 1. An overview of how interactions between C. albicans and Gram-positive members of the human flora may influence disease.
(A) S. gordonii, a normal colonizer of the oral cavity, enhances C. albicans survival and persistence, thus contributing to the development of C. albicans infections. In this environment, S. gordonii adhere to cells by expressing a complex repertoire of cell surface polypeptides (AgI/II) (a) that recognize a range of host tissue proteins and cellular receptors [7]. After bacterial attachment, C. albicans can selectively attach to surface-bound bacteria by means of protein-protein interactions [10], [12] or by direct recognition of salivary proteins (basic proline-rich proteins, bPRPs) previously adsorbed by S. gordonii cells [11] (b). Coaggregation of the bacterium and C. albicans contributes to biofilm formation and results in closer proximity for cell-cell communication. Through a diffusible signal (DS) molecule (c), S. gordonii suppresses farnesol-mediated inhibition of hypha formation, thereby enhancing the potential for C. albicans to form biofilms and thus its ability to invade tissue [7]. In addition, Streptococcus promotes (d) fungal growth by secreting metabolic products that can be used as a carbon source by C. albicans [1]. Likewise, C. albicans enhances the survival and colonization of S. gordonii by reducing the oxygen tension to levels preferred by streptococci and by providing bacterial growth stimulatory factors as a result of nutrient metabolism [1], [11]. These favorable conditions promote the formation of mature fungal-bacterial biofilms surrounded by an extracellular matrix, to which (e) other bacterial or fungal species can bind. These interactions may make oral infections more persistent and harder to treat. (B) Lactobacillus sp., which normally inhabits the female reproductive tract, defends the host against colonization of pathogens such as C. albicans. Evidence suggests that the bacterium reduces the adhesion of C. albicans to epithelial cells either by (a) outcompeting fungal cells for adhesion sites, such as cellular receptors to which Lactobacillus has higher affinity, or (b) by secreting biosurfactants such as surlactin that physically decrease fungal binding. Most Lactobacillus strains release (c) hydrogen peroxide (H2O2) and (d) lactic acid or other fatty acids that inhibit C. albicans proliferation and invasive hypha formation. Bacteriocin-like substances (e) produced by Lactobacillus suppress the fungal growth to directly decrease its load [8].