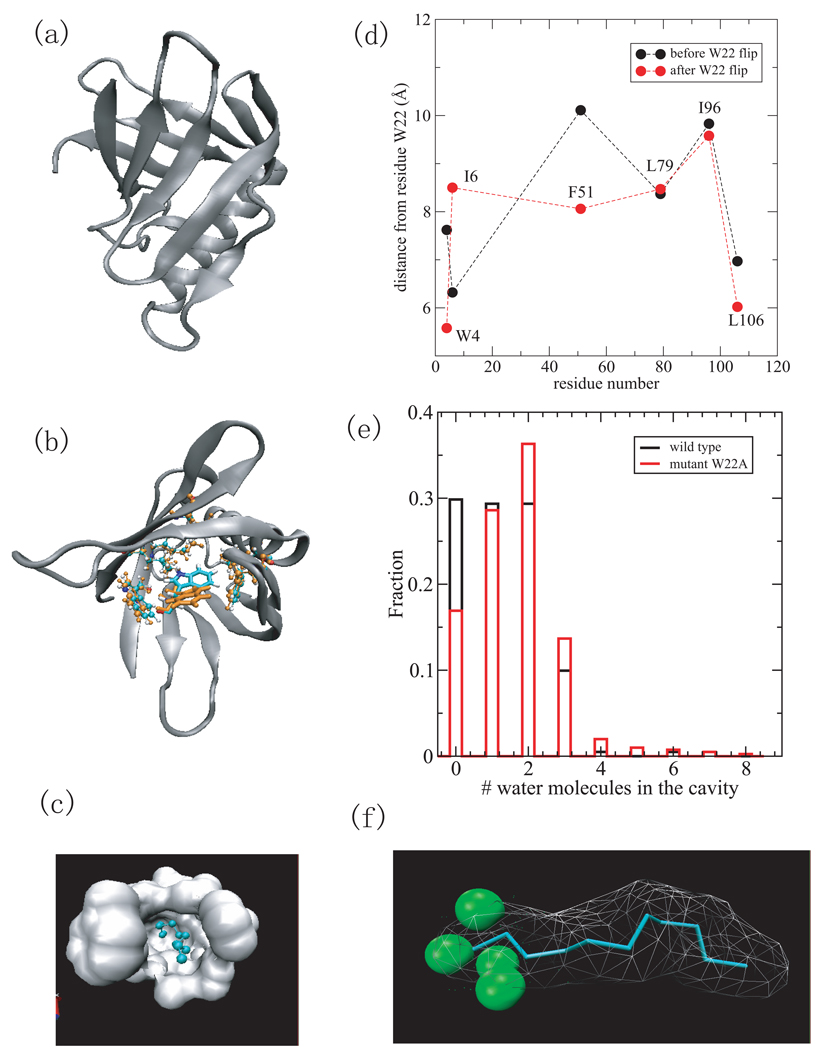
Fig.2.
(a) A cartoon representation of the MxiM protein (PDB ID 1y9l) with β-structure and helical domains. (b) The structures of the residues lining the cavity including W4, I6, W22, F51, L79, I96, and L106 at 0ps (cyran) and 550ps (orange), respectively. Residue W22 is in licorice representation and others are in cpk. Only the side chain of W22 has a large reorientation. (c) Surface representation of the hydrophobic cavity where the ligand, detergent acyl chain is located. (d) The distance between residues (W4,I6,F51,L79,I96,and L106) from W22 versus the residue number before the W22 flip (black) and after the W22 flip (red). (e) The histogram of the number of water molecules within 2 Å of the ligand for wild-type MixM (black) and mutant W22A (red). (e) The density of water within 2Å of ligand. The green spheres indicate water density larger than 1/2 of bulk density.