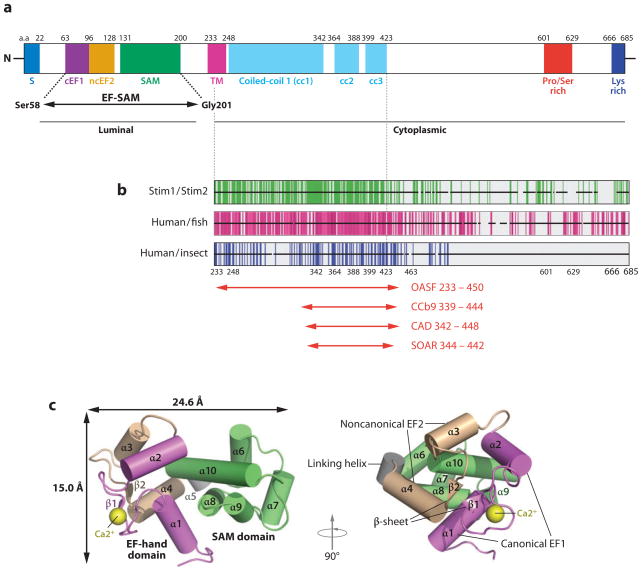
Figure 2.
Structure and properties of STIM1. (a) Domain structure of human STIM1 (adapted with permission from Reference 27). Shown are the signal peptide (S), the canonical EF-hand 1 (cEF1), the noncanonical EF-hand 2 (ncEF2), the SAM (sterile α-motif ) domain, the transmembrane domain (TM), three predicted coiled-coil regions (cc1, cc2, and cc3), the proline- and serine-rich region, and the lysine-rich (polybasic) region at the C terminus. The EF-SAM fragment whose structure was determined by NMR spectroscopy is indicated. The region to the left of the TM is located in the ER lumen, whereas the region to the right is located in the cytoplasm. Residue numbers at the approximate boundaries of the domains are indicated above the diagram. Coiled-coils cc1 and cc2 have long been recognized in STIM proteins (97, 98, 105) and are assigned high probability in STIM1 by COILS; the predicted coiled-coil cc3 is assigned a low probability by COILS in STIM1, but a relatively high probability in Aedes aegypti Stim and Anopheles gambiae Stim, and in STIM2 when core hydrophobic positions are weighted. The existence and precise boundaries of cc3 require experimental confirmation. (b) Sequence conservation in the STIM C-terminal region. Each horizontal black bar represents the human STIM1 sequence, with gaps introduced as necessary to maintain alignment with human STIM2, fish STIM1 orthologs, or insect Stim proteins, as indicated. Vertical green lines indicate identity of the human STIM1 residue with the residue at the corresponding position of human STIM2; vertical magenta lines indicate identity of the human STIM1 residue with residues at the corresponding position in at least four of five fish orthologs; vertical blue lines indicate identity with residues in at least two of three insect Stim proteins. Adapted from Reference 36. (c) Structure of the EF-SAM fragment deduced by NMR spectroscopy (adapted with permission from Reference 27). Alpha-helices are depicted as cylinders. (The canonical EF-hand 1 is magenta, the noncanonical EF-hand 2 is beige, and the SAM domain is green; the Ca2+ ion bound to EF-hand 1 is a yellow sphere.) Two views related by a 90° rotation are shown.