Figure 6. Protection is not linked to differential entry of the toxin.
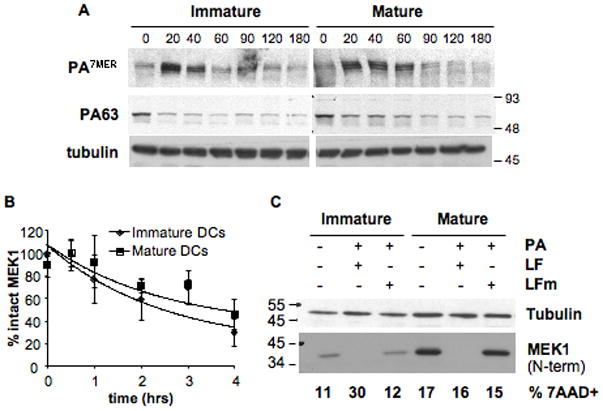
B6 derived DCs were matured over-night with LPS or left immature and a binding assay was performed (A,B): cells were incubated with 500ng/ml of trypsin-nicked PA (Abrami et al., 2003) and 200ng/ml LF, for 1 h on ice. Then washed with toxin free media and transferred at 37°C for different periods of time (in min.). 40 μg of total cell extracts were analyzed by western blotting to detect PA (A), LF processed MEK1 (anti Nterm antibody) and total MEK1 (anti Cterm antibody) (B, see also Suppl. Fig. 4). Equal loading is assessed by an antibody anti-tubulin. B) The amount of intact MEK1 (MEK1 Nterm on Suppl. Fig. 4) was quantified by densitometry, normalized to the amount of total MEK1 (detected with the antibody anti Cterm) as loading control, and the resulting values were normalized to the amount of intact MEK1 (Nterm) at time t=0. The kinetics of decrease of intact MEK1 were compared for immature and mature DCs. Data is expressed as mean±SD from 4 independent experiments. C) DCs from B6 mice were matured by 8 h LPS treatment or left immature, and treated for 24 h with 500ng/ml PA and 20ng/ml LF or LFm (E687C inactive mutant). 40μg of each cell extract were analyzed by western blot with an antibody against the Nterm part of MEK1 to detect only intact MEK1. Equal loading was assessed with an anti-tubulin antibody. An aliquot of the same cells was stained with 7AAD to determine the amount of dead cells by flow cytometry, indicated as % within the CD11c+ population. Blots from one representative experiment (n≥3).
